Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis include Granulomatosis with Polyangiitis (GPA), Microscopic Polyangiitis (MPA), and Eosinophilic Granulomatosis with Polyangiitis (EGPA). EGPA presents a different pathogenic, clinical, and therapeutic entity. However, international therapeutical recommendations (ACR, EULAR and KDIGO), epidemiological studies and clinical trials (RAVE and RITUXVAS) (1-4) include MPA and GPA as a combined group. Cyclophosphamide (CYC) and Rituximab (RTX) are considered the first-line therapeutic option in MPA and GPA. RAVE and RITUXVAS trials have demonstrated similar efficacy and safety with CYC and RTX. However, these studies are often limited in follow-up time, which may influence on the results.OBJECTIVESTo compare safety and relapse rates between CYC and RTX.
Methods: Observational study of 196 patients diagnosed with ANCA-associated vasculitis at a referral hospital in northern Spain from January 1, 2000, to December 31, 2024. Classification of ANCA vasculitis was based on the 2022 ACR/EULAR criteria. Depending on the induction therapy administered, patients were divided into two groups: i) Patients treated exclusively with CYC and ii) with RTX. Patients who received both treatments at any time during follow-up were excluded. Rates of Adverse effects are expressed as the exposure-adjusted incidence rate (EAIR) per 100 person years (PY).
Results: A total of 87 patients were finally included (44 women/43 men); 48 received CYC and 30 received RTX, mean ages at diagnosis of 65.7±13.4 and 57.4±14.8 years, respectively. MPA was the most frequent vasculitis subtype in both groups. Most clinical and laboratory features were similar, except for renal and abdominal involvement that was more common in the CYC group (Table 1).Despite a shorter mean follow-up in patients in the CYM group (17.4±23 vs 31.5±26.1 months), they present significantly more serious adverse events (83.3% vs. 56.4%; p= 0.0085) EAIR of 4.78 /100 PY Vs 1.78/100 PY. The most frequent adverse effect was severe infections (58% vs. 33.3%; p= 0.0304) with a EAIR of XX/100 PY on the CYP group Vs a EAIR of XX/ 100 PY on the RTX group. (Table 2).No significant differences were found for other adverse events. During follow-up, relapses occurred more frequently in the CYC group (14% vs 5.1%; p= 0.178) with an EAIR of 0.8 and 0.16 respectively. (Table 2).
Conclusion: In our series, patients treated with CYC had statistically more frequently severe infections and a trend to higher relapse rates.
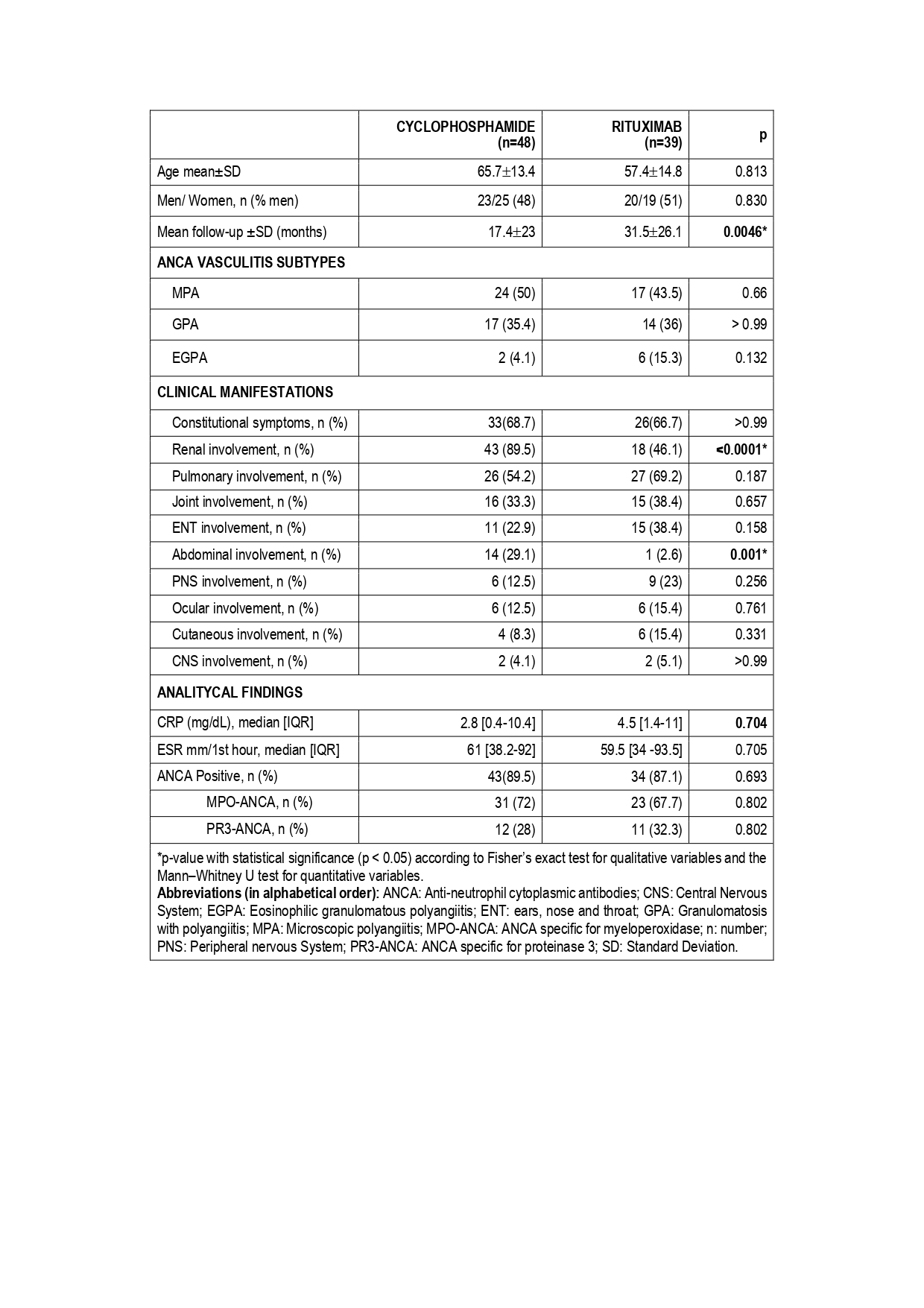 TABLE 1. Clinical characteristics and analytical findings of 87 patients with ANCA associated Vasculitis treated with CYC and RTX. Data were obtained at moment of diagnosis.
TABLE 1. Clinical characteristics and analytical findings of 87 patients with ANCA associated Vasculitis treated with CYC and RTX. Data were obtained at moment of diagnosis.
.jpg) TABLE 2. Adverse Events and Relapse in 87 patients with ANCA associated Vasculitis treated with CYC and RTX.
TABLE 2. Adverse Events and Relapse in 87 patients with ANCA associated Vasculitis treated with CYC and RTX.
To cite this abstract in AMA style:
Benavides Villanueva F, Prieto-Peña D, Calvo-Río V, Renuncio-García M, Martin-Gutierrez A, Sanchez-Lopez A, Poo-fernandez C, Rodríguez-Vidriales m, Blanco R. Cyclophosphamide versus Rituximab in the treatment of Anca-associated Vasculitis: Adverse events and Relapses [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/cyclophosphamide-versus-rituximab-in-the-treatment-of-anca-associated-vasculitis-adverse-events-and-relapses/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cyclophosphamide-versus-rituximab-in-the-treatment-of-anca-associated-vasculitis-adverse-events-and-relapses/
