Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Treatment guidelines recommend tumor necrosis factor inhibitors (TNFi) and interleukin-17A inhibitors (IL-17Ai) as options for patients with PsA or axSpA with persistent disease activity despite conventional treatment (1-3). TNFi’s are commonly used first-line treatment in PsA and axSpA (4). There is little evidence available to inform selection of therapy after failure with TNFi treatment. This study compares the effectiveness of second TNFi (cycling) versus switching to IL-17Ai among patients with PsA and axSpA who discontinued a first TNFi.
Methods: This study included patients with a diagnosis of PsA or axSpA enrolled in the CorEvitas PsA/SpA Registry who initiated a second TNFi or IL-17Ai after discontinuation of a first TNFi. Patients were stratified into two cohorts, those who: 1) initiated a second TNFi (cyclers) and 2) initiated an IL-17Ai (switchers). The primary outcome among the PsA group was change from baseline (CFB) in cDAPSA and among the axSpA group was CFB in BASDAI, at 6-month follow-up. Linear regression models, for continuous outcomes, or Poisson regression models (5), for binary outcomes, were fit to each primary and secondary outcome to compare switching versus cycling after 6-month follow-up visit, using the inverse probability of treatment (IPT) weighted approach to account for imbalance in baseline covariates.
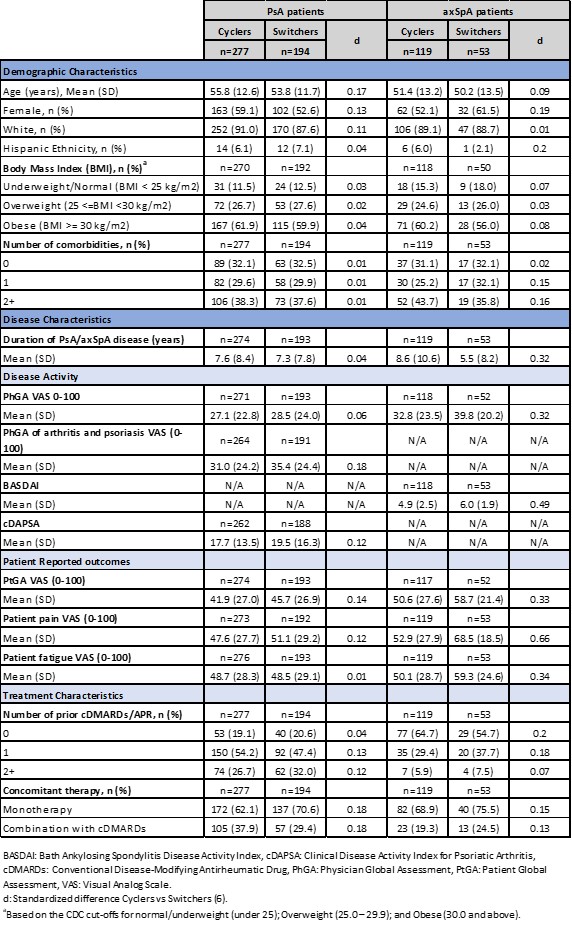
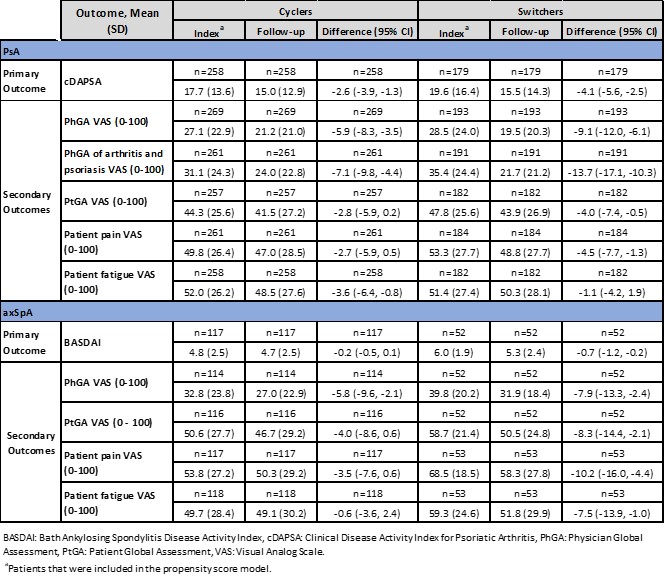
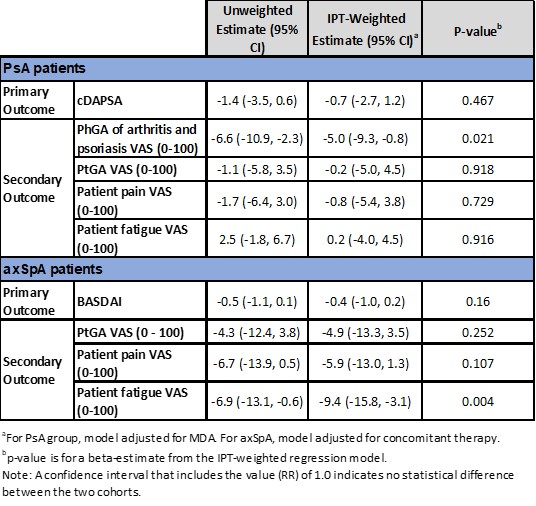
Results: The PsA cohort included 277 (59%) and 194 (41%) and axSpA included 119 (69%) and 53 (31%), cyclers and switchers respectively. Demographic and disease characteristics were largely comparable between groups for PsA and axSpA cohorts. Disease activity and PROs were generally higher in switchers; treatment characteristics were largely similar between both cohorts (Table 1). Overall, for PsA and axSpA cohort at 6-month follow up, switchers demonstrated numerically greater improvements compared to cyclers for both primary and secondary outcomes (Table 2). For IPT-weighted estimates, in the PsA cohort, switchers demonstrated a 0.7-point improvement in the primary outcome (95% CI: -2.7, 1.2; p=0.467) compared to cyclers, and in the axSpA cohort switchers displayed a 0.4-point improvement (95% CI: -1.0, 0.2, p=0.160) (Table 3). In the PsA cohort, PhGA of arthritis and psoriasis switchers had statistically significantly greater improvements compared to cyclers (-5.0; 95% CI: -9.3, -0.8, p=0.021). Similarly, in the axSpA cohort, improvement in patient fatigue was significantly higher in the switchers compared to cyclers (-9.4; 95% CI: -15.8, -3.1, p=0.004) (Table 3).
Conclusion: Findings from this real-world analysis suggest that switching from a TNFi to an IL-17Ai results in similar or numerically improved outcomes compared to cycling to a subsequent TNFi in both PsA and axSpA cohort. While additional research is needed, these results underscore the importance of considering alternate mechanisms of action as possible suitable options for patients discontinuing a first TNFi.
1. Coates, L C, Nat. Rev. Rheumatol., 18 (2022) 465.
2. Ramiro, S., Ann. Rheum. Dis., 82 (2023) 19.
3. Gossec, L., Ann. Rheum. Dis., 83 (2024) 706.
4. Caporali, R., Mod. Rheumatol, 34 (2024) 11.
5. Zou, G., Am. J. Epidemiol., 159 (2004) 702.
6. Austin, P.C., Statist. Med. 28 (2009) 3083.
To cite this abstract in AMA style:
Ogdie A, Middaugh N, Marchese M, Walsh J, Bello N, Lisse J, Kronbergs A, Grace E, Ngantcha M, Mease P. Cycling to TNFi vs. Switching to IL-17Ai After a First TNFi Discontinuation Among Patients with PsA and axSpA: The CorEvitas PsA/SpA Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cycling-to-tnfi-vs-switching-to-il-17ai-after-a-first-tnfi-discontinuation-among-patients-with-psa-and-axspa-the-corevitas-psa-spa-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cycling-to-tnfi-vs-switching-to-il-17ai-after-a-first-tnfi-discontinuation-among-patients-with-psa-and-axspa-the-corevitas-psa-spa-registry/