Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) is a validated outcome measure designed to assess cutaneous lupus erythematosus (CLE) activity (CLASI-A) and damage (CLASI-D). Scoring is separated by anatomic site, with greater weight assigned to the head and neck. CLASI-A specifically assesses erythema, scale, alopecia, and mucous membrane involvement, with maximum scores of 39, 26, 4, and 1, respectively. There is little data depicting how CLASI-A erythema and scale scores change over time in relation to total CLASI-A scores. Thus, we designed a prospective observational CLE patient cohort study that further elucidates trends in erythema and scale to better understand disease course. Overall, we hypothesize that CLASI-A erythema and scale scores trend closely and contribute similar changes in total CLASI-A.
Methods: 130 CLE patients on standard-of-care therapies were followed at regular two-month intervals over 6 months. Patients were recruited from three outpatient dermatology clinics from July 2018 to May 2023. CLE disease activity was assessed using the Cutaneous Lupus Disease Area and Severity Index (CLASI). The primary and secondary outcomes were the mean change and mean percent change (MPC) of total CLASI-A, CLASI-A erythema, and CLASI-A scale scores between baseline and each follow-up visit. Predictive variables associated with disease activity trends were analyzed with linear mixed-effects modeling adjusted for baseline CLASI scores, time, and within-subject correlation.
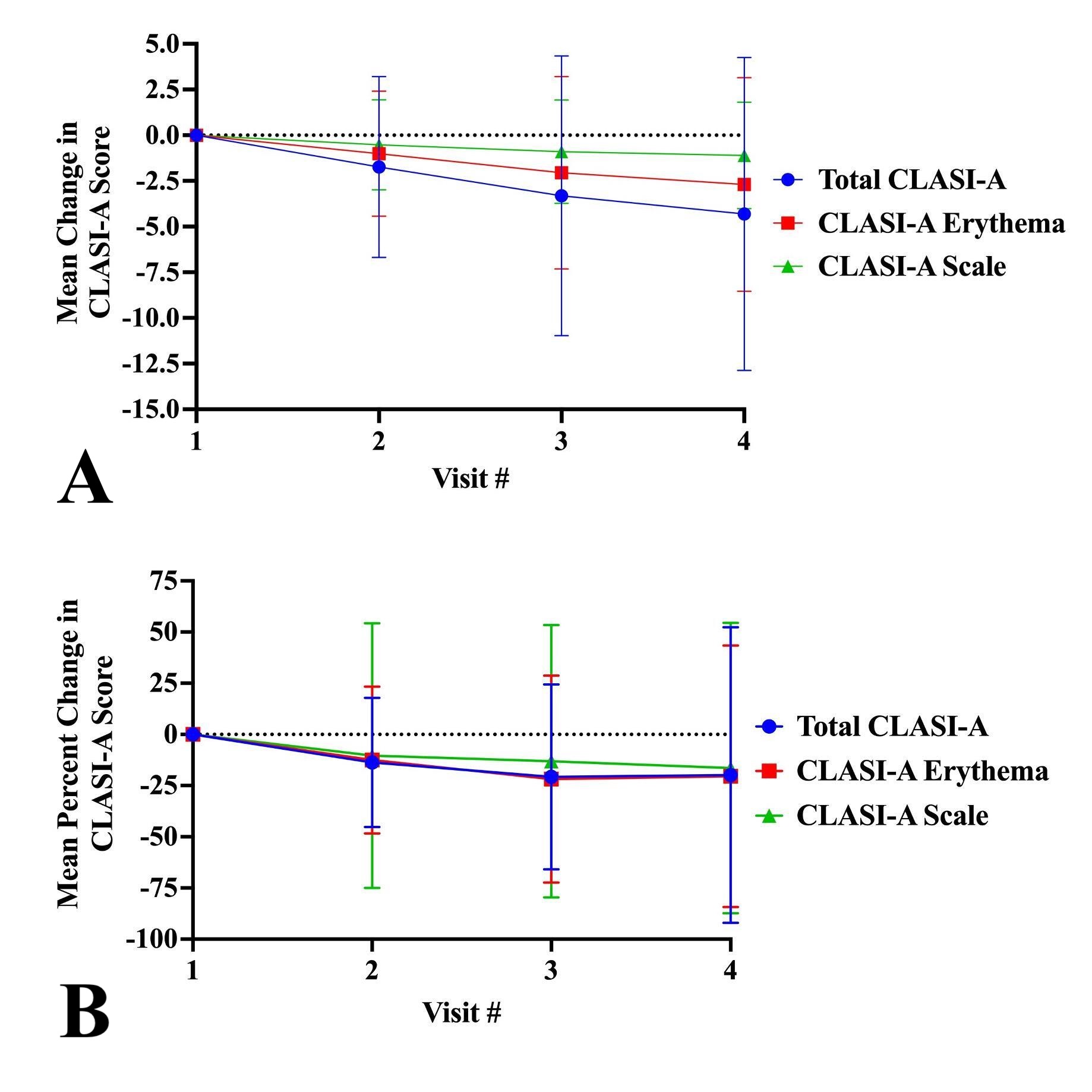
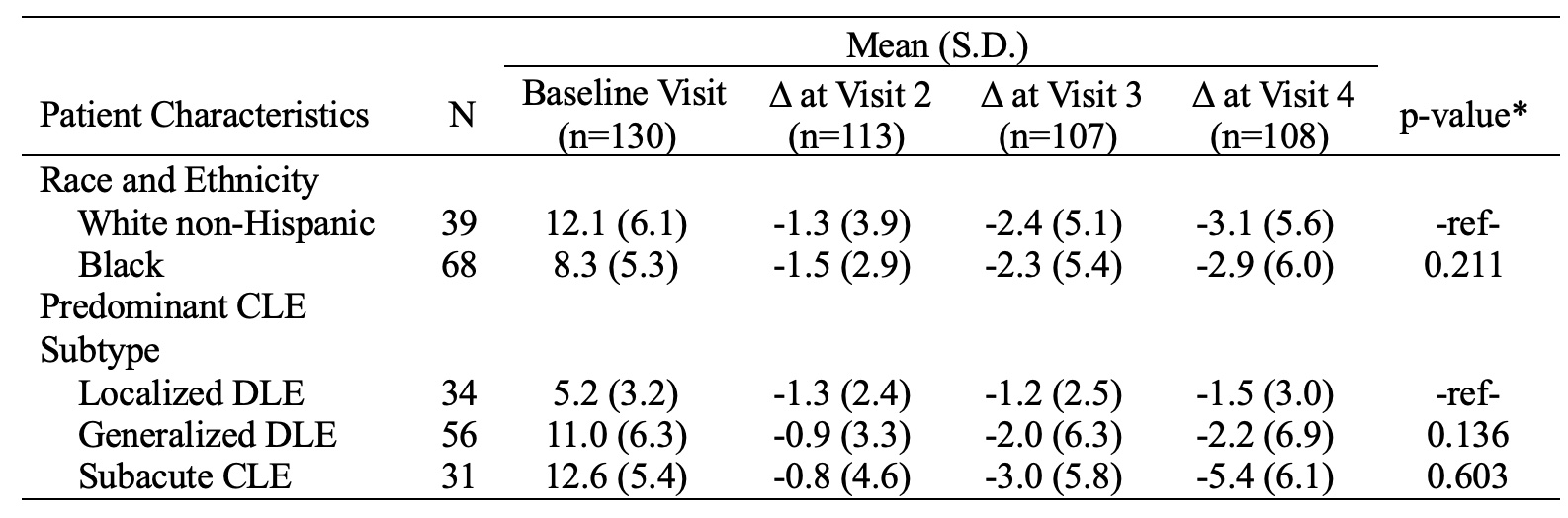
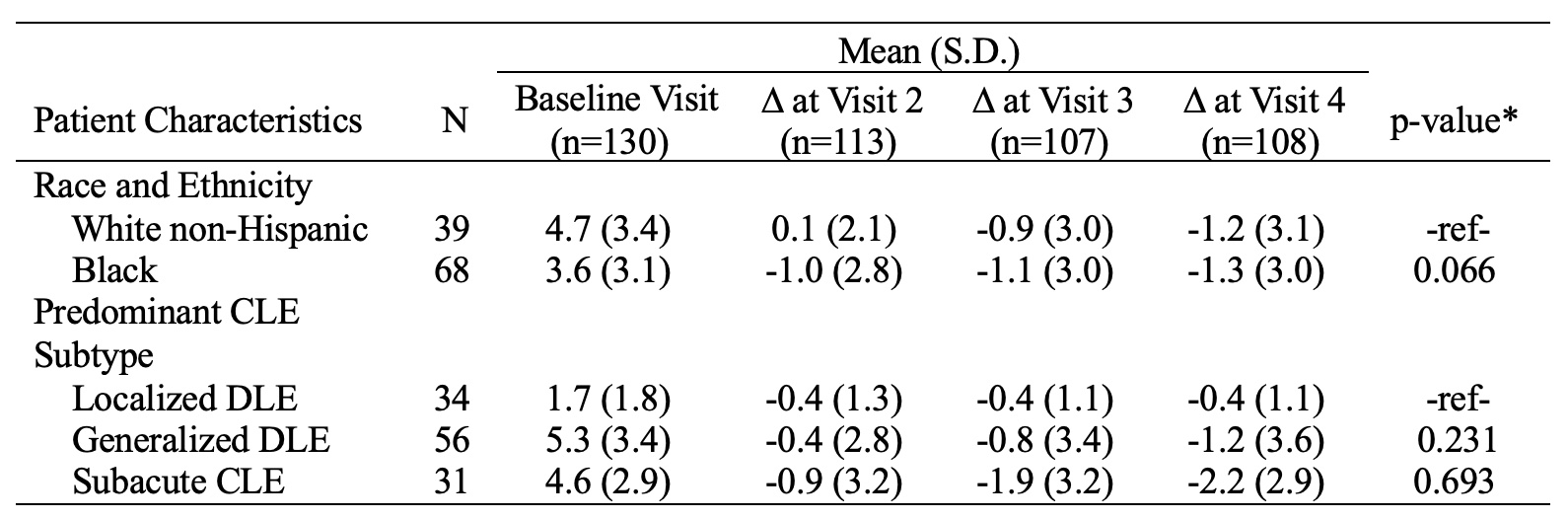
Results: The mean changes in CLASI-A scores over six months were -4.3±8.6 for total CLASI-A, -2.7±5.9 for CLASI-A erythema, and -1.1±2.9 for CLASI-A scale. The MPCs in total CLASI-A, CLASI-A erythema, and CLASI-A scale were -19.8%±72.0%, -20.4%±63.9%, and -16.4%±71.0%, respectively (Figure 1). No significant differences existed between the MPC of CLASI-A erythema and scale (p=0.651). Mean changes of CLASI-A erythema and scale at visit 4 were -3.1±5.6 and -1.2±3.1 for White Non-Hispanic patients (n=39), -2.9±6.0 (p=0.211) and -1.3±3.0 (p=0.066) for Black patients (n=68), -1.5±3.0 and -0.4±1.1 for localized discoid lupus erythematosus (DLE) (n=34), -2.2±6.9 (p=0.136) and -1.2±3.6 (p=0.231) for generalized DLE (n=56), and -5.4±6.1 (p=0.603) and -2.2±2.9 (p=0.693) for subacute CLE (n=31), respectively (Tables 1-2).
Conclusion: CLASI-A erythema and scale trend similarly over 6 months and are well represented by changes in total CLASI-A, highlighting CLASI-A’s reliability. Furthermore, erythema and scale trends were not impacted by either race and ethnicity or CLE subtype, emphasizing the CLASI’s sensitivity to capture changes in activity across varying skin pigmentations and CLE subtypes and further underscoring its utility for use in clinical trials.
To cite this abstract in AMA style:
Cepica T, Xie L, Faden D, Stone C, Werth V, Chong B. Cutaneous Lupus Erythema and Scale Have Similar Six-month Trends Without Significant Impact from Race/ethnicity or Disease Subtype [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cutaneous-lupus-erythema-and-scale-have-similar-six-month-trends-without-significant-impact-from-race-ethnicity-or-disease-subtype/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cutaneous-lupus-erythema-and-scale-have-similar-six-month-trends-without-significant-impact-from-race-ethnicity-or-disease-subtype/