Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Vaccination is fundamental to reduce COVID-19 risk and its complications. Vaccine hesitancy is a threat to COVID-19 vaccination uptake. We assessed the frequency of COVID-19 vaccine hesitancy among RD and other patients.
Methods: Between Nov-Dec 2020 (prior to COVID-19 vaccine roll-out), a cross-sectional survey was completed by patients presenting to a Canadian university-affiliated hospital network for influenza immunization. Sociodemographics, comorbidities, concomitant medications, previous COVID-19 infection, and perceptions related to COVID-19 vaccination (e.g., likelihood of receiving a future COVID-19 vaccine, causes of vaccine hesitancy) were assessed. We defined 3 specific groups: (i) RD, (ii) non-RD immunosuppressed patients (HIV, cancer, transplant, inflammatory bowel disease), and (iii) other. According to self-reported likelihood to receive a future COVID-19 vaccine and the tertile distribution of the responses, patients were classified as significantly hesitant (scores 0-7); somewhat hesitant (scores 7.5-9.5), or non-hesitant (score 10). Logistic regression using backward stepwise variable selection was performed to evaluate determinants of vaccine hesitancy.
Results: Among 2491 people vaccinated, 1793 people completed the survey (72%). Of those, 820 were patients (45.7%), 542 physicians or nurses (30.2%), 228 patients’ family members (12.7%), and 203 other (11.3%). Participants had a mean age (±SD) of 52.2±16.9 years, and 58.6% were females. Just over one-tenth (10.7%, n=191) had RD, 9.4% (n=169) were immunosuppressed non-RD patients, and the remaining were other participants. Concerning COVID-19 vaccination, most subjects were non-hesitant (n=1124, 62.7%), 315 were somewhat hesitant (17.6%), and 354 (19.7%) significantly hesitant. RD patients were significantly more hesitant than other groups (hesitant %: RD: 44.5 vs. non-RD immunosuppressed: 30.8 – OR:1.80, 95% CI: 1.17 – 2.78; RD: 44.5 vs. other: 37.1 – OR: 1.36, 95% CI: 1.00 – 1.84). Vaccine hesitancy was associated with the following: perception that COVID-19 vaccines should not be mandatory; having concerns about vaccine safety; and being uncertain if vaccine benefits overweight its risks. Table 1 for predictors of vaccine hesitancy.
Conclusion: COVID-19 vaccine hesitancy was common among RD patients receiving influenza vaccination. Vaccine hesitancy in RD was higher than in other immunosuppressed patients and the general population. Factors associated with vaccine hesitancy are not unique to RD. Education about the benefits and safety of COVID-19 vaccines might enhance vaccine uptake among RD patients.
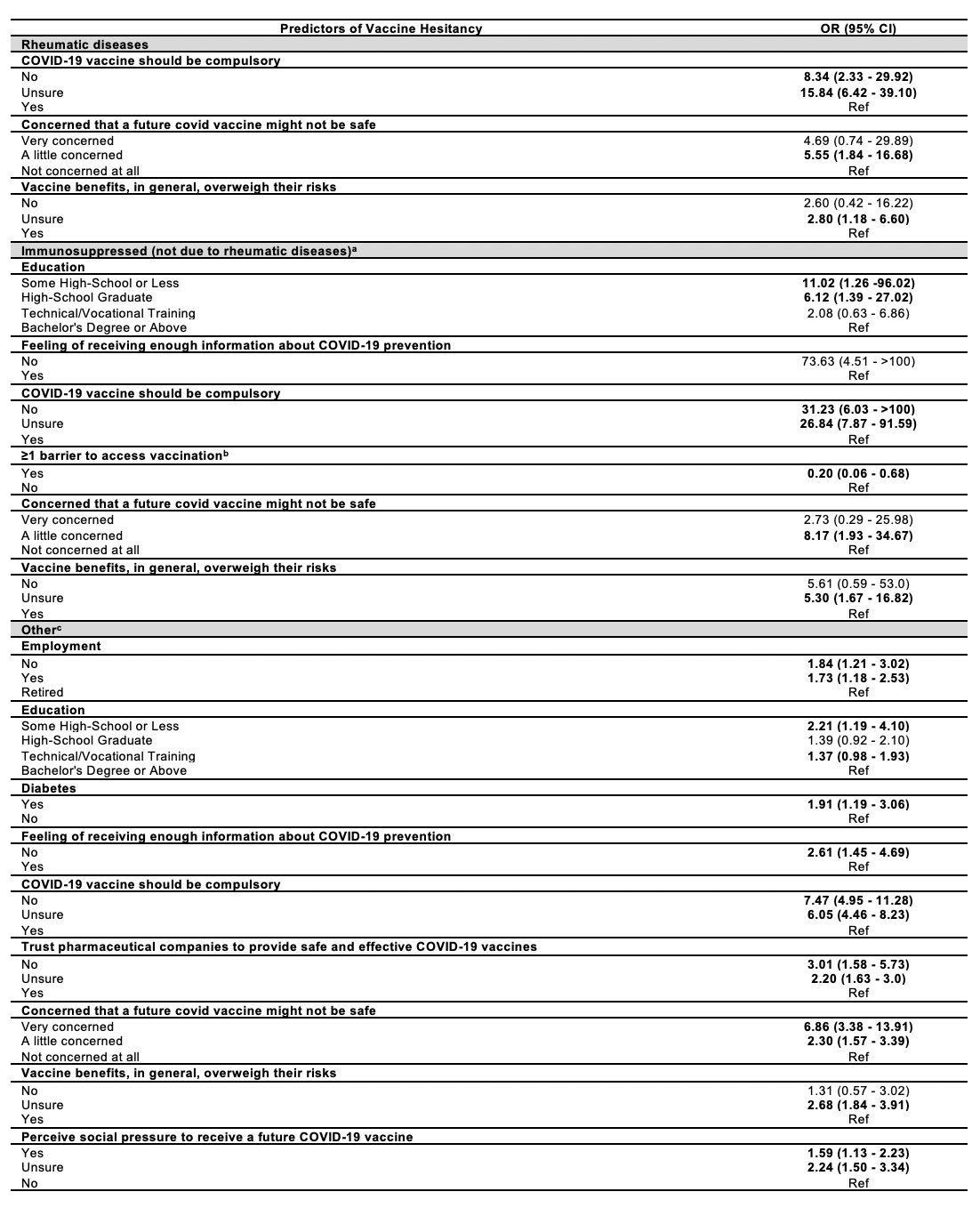 Table 1. Factors associated with COVID_19 vaccine hesitancy among people receiving influenza vaccine (no hesitancy as a reference group); (a) HIV, transplant, cancer, and inflammatory bowel disease; (b) Distance to the vaccine provider, time needed to get to the vaccine provider, waiting time at the vaccine provider, cost/parking in getting to the vaccine provider, and efforts of traveling to the vaccine provider; (c) Participants that were not receiving immunosuppressant treatment or did not have a disease that affects the immune system.
Table 1. Factors associated with COVID_19 vaccine hesitancy among people receiving influenza vaccine (no hesitancy as a reference group); (a) HIV, transplant, cancer, and inflammatory bowel disease; (b) Distance to the vaccine provider, time needed to get to the vaccine provider, waiting time at the vaccine provider, cost/parking in getting to the vaccine provider, and efforts of traveling to the vaccine provider; (c) Participants that were not receiving immunosuppressant treatment or did not have a disease that affects the immune system.
To cite this abstract in AMA style:
Valerio V, Rampakakis E, Hudson M, Bernatsky S, Hazel E, Colmegna I. COVID-19 Vaccine Hesitancy Among Patients with Rheumatic and Other Diseases [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/covid-19-vaccine-hesitancy-among-patients-with-rheumatic-and-other-diseases/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-vaccine-hesitancy-among-patients-with-rheumatic-and-other-diseases/
