Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: COVID-19 vaccination in autoimmune diseases (COVAD) study is a large-scale real-world data on COVID-19 vaccine safety in autoimmune rheumatic diseases (AIRDs) including rheumatoid arthritis (RA). The COVAD study aimed to assess COVID-19 vaccination-related adverse events (AEs) till seven days post-vaccination in RA patients.
Methods: COVAD study group comprised >110 collaborators across 94 countries. The Study was conducted from March 2021 to December 2021. Survey monkey platform-based self-reported online survey captured the data related to COVID-19 vaccination-related AEs in RA, other AIRDs, other (non-rheumatic) autoimmune diseases (nrAIDs), and healthy controls (HCs). Active and inactive disease were patient self-reported and decided based on the need for hiking immunosuppression prior to vaccination. Descriptive and multivariable regression analyses adjusting for age, gender, ethnicity, vaccine type, and disease modifying anti-rheumatic drugs (DMARDs) were performed. Statistically significant results following multivariate regression are reported.
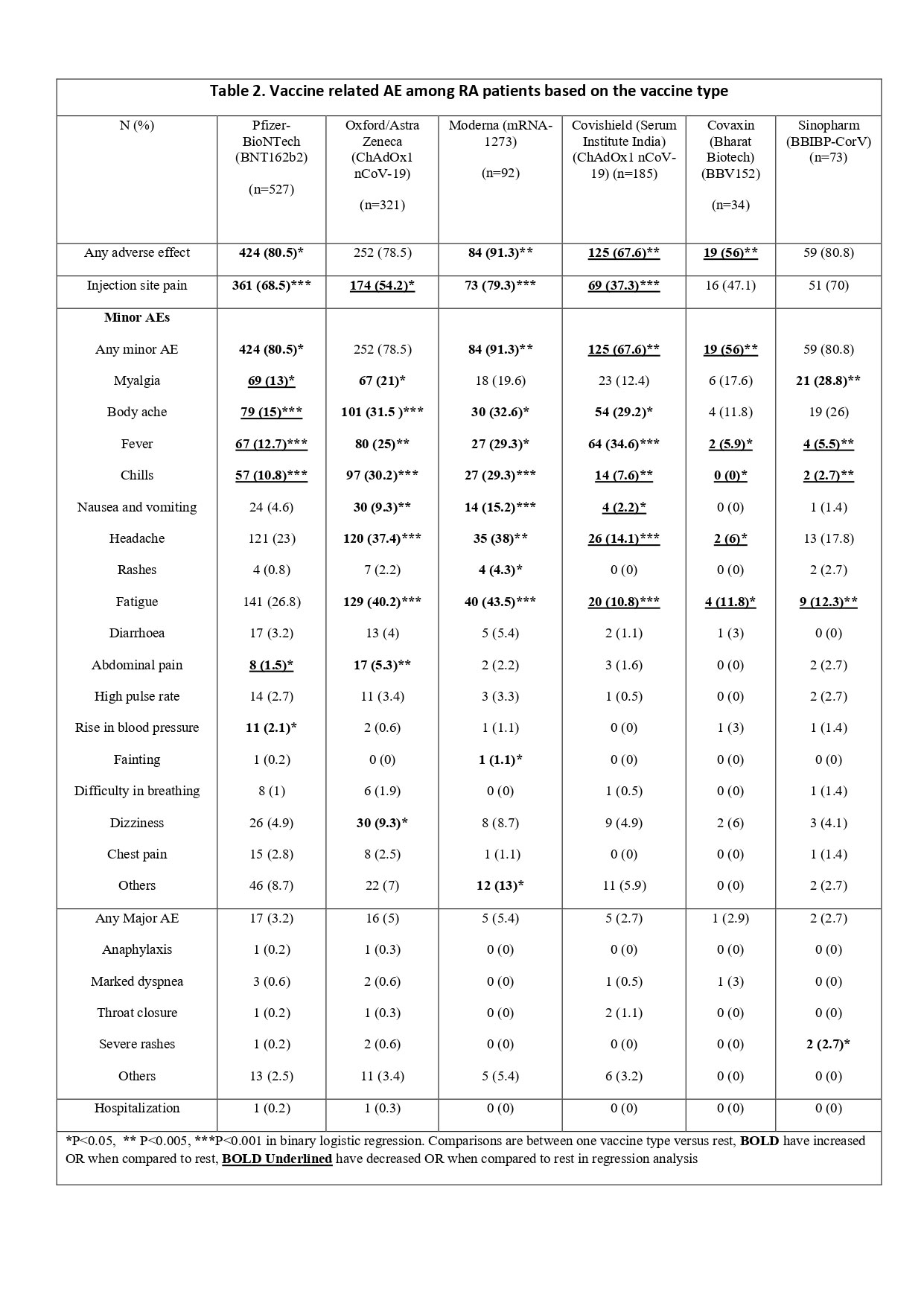
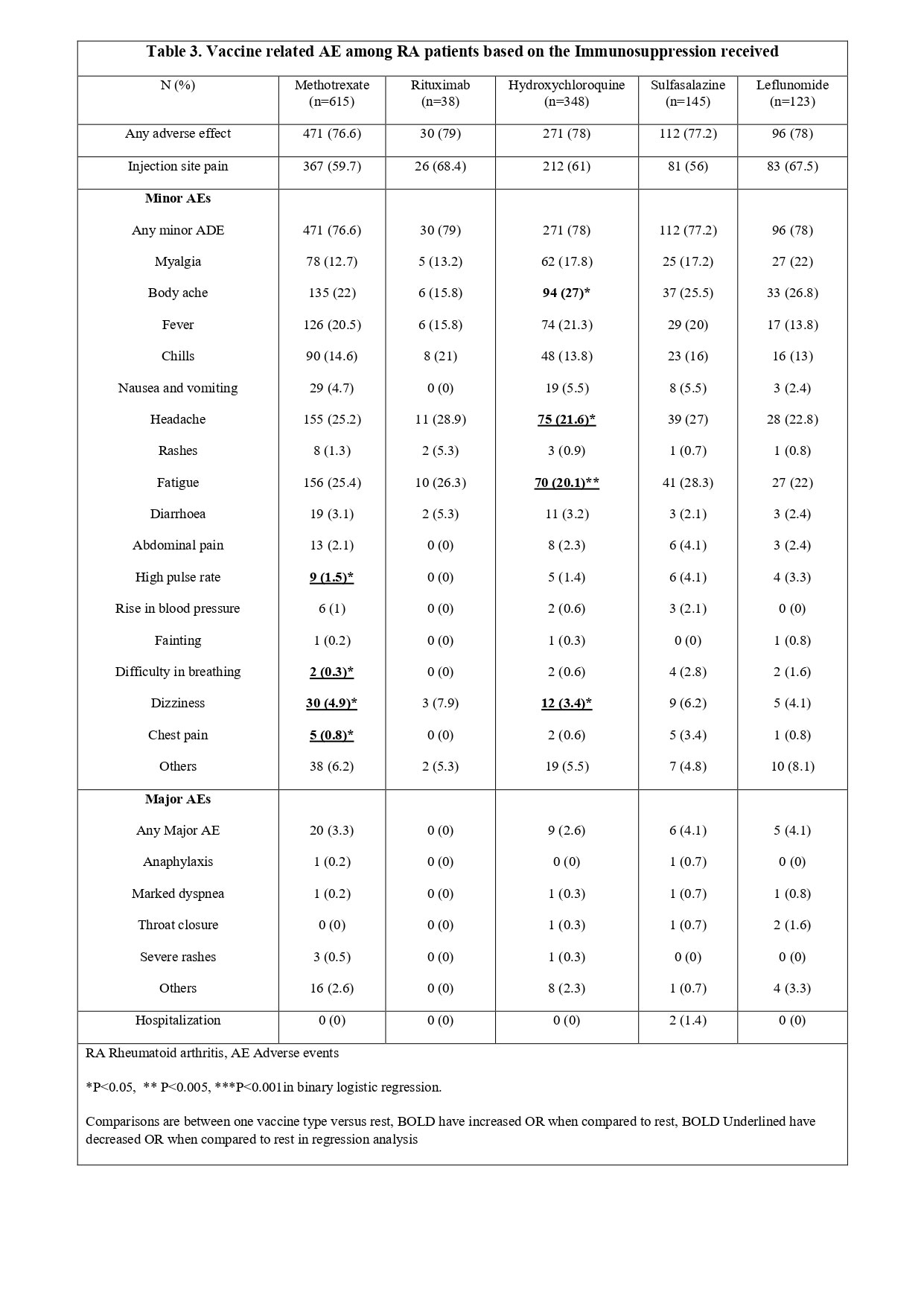
Results: Of the 9462 total complete respondents, 14% (n=1347) were RA patients, with a mean (SD) age 50.7 (13.7) years, 87% were females, 56% were Caucasian, and 71% had completed 2 doses. BNT162b2 (Pfizer) (39.1%) and ChAdOx1 nCoV-19 (Oxford)(23.8%) were the most common vaccines received (Table 1). Overall, 77.1% of RA patients reported AEs (77.1%-minor and 4.2% -major). Active RA had similar AEs and hospitalization compared to inactive RA. Between the different vaccines, mRNA 1273 (Moderna) recipients had reported highest overall AEs, minor AEs [OR for both 3.1 (1.4-6.6), p 0.003], and injection site pain [OR 2.5 (1.5-4.3), p< 0.001]. Similarly, Pfizer recipients too reported higher overall AE, minor AEs [OR for both 1.3 (1.03-1.8), p 0.027], and injection site pain [OR 2.1 (1.5-2.5), p< 0.001] compared to the rest. Oxford recipients had higher frequencies of myalgia, fever, chills, nausea/vomiting, headache, and fatigue compared to the rest of vaccine recipients (Table 2). ChAdOx1 nCoV-19 (Covishield), BBV152 (Covaxin), and BBIBP-CorV (Sinopharm) recipients had reported lower AEs compared to the rest. RA patients on different DMARDs reported similar AEs except for patients on methotrexate and hydroxychloroquine who reported fewer minor AEs (Table 3). When compared to HC and AIRDs, RA patients reported similar overall AEs, injection site pain, overall minor AEs, and hospitalizations, except for higher frequencies of dizziness compared to HC [OR 1.4 (1.1-2), p 0.010], and lower frequencies of fatigue [OR 0.8 (0.6-0.9), p 0.015] compared to AIRDs. When compared to other nrAIDs, RA reported lower overall AEs [OR 0.7 (0.5-0.9), p 0.017], injection site pain [OR 0.6 (0.5-0.8), p 0.002] and minor AEs [OR 0.7 (0.5-0.9), p 0.017]. Major AEs and hospitalization were similar between RA, AIRDs, nrAIDs and HC.
Conclusion: Despite differences in the frequencies of AEs between different COVID-19 vaccines, all were largely well tolerated in RA patients. Frequencies of AEs were no different between active versus inactive RA. The findings of the present study provide reassurance on safety of COVID-19 vaccination in RA.
To cite this abstract in AMA style:
R N, Parodis I, Joshi M, Sen P, Kim M, Agarwal V, Kardes S, Lilleker J, Chinoy H, Distler O, Tan A, Shinjo S, Salim B, Gheita T, Ziade N, Velikova T, Chatterjee T, Nune A, Milchert M, Gracia-Ramos A, O’Callaghan A, Saavedra Salinas M, Cavagna L, Kuwana M, Knitza J, Day J, Makol A, Pauling J, Wincup C, Zamora Tehozol E, Rojas Serrano J, Garcia-De La Torre I, Aggarwal R, Agarwal V, Gupta L, Nikiphorou E, Study Group C. COVID-19 Vaccination in Autoimmune Diseases Study: Vaccine Safety in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/covid-19-vaccination-in-autoimmune-diseases-study-vaccine-safety-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-vaccination-in-autoimmune-diseases-study-vaccine-safety-in-rheumatoid-arthritis/