Session Information
Date: Saturday, November 6, 2021
Title: Epidemiology & Public Health Poster I: COVID-19 & Vaccination (0084–0117)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Over 135 million Americans were fully vaccinated to COVID-19 by June 2021, yet there was a paucity of data on side effects for those with autoimmune conditions commonly seen in rheumatology. Side effects can prevent recipients from receiving a second vaccination and can be related to the immunogenicity of the vaccine and/or recipient. The purpose of our study was to characterize the rates and types of COVID-19 vaccine side effects in adults with rheumatic diseases and assess if there were associations with disease treatment, severity, or vaccine type.
Methods: Data were provided by adults enrolled in FORWARD, The National Databank for Rheumatic Diseases. Participants complete comprehensive semiannual questionnaires and were invited to answer supplemental questionnaires focused on COVID-19 vaccination in March and April 2021. Only individuals who received Pfizer-BioNTech or Moderna vaccines were included in this study due to insufficient sample sizes for other vaccines. Vaccinated respondents were compared by whether they reported experiencing side effects following vaccination and by vaccine type, with significance (p < 0.05) assessed by Student’s t-tests and chi-squared tests, as appropriate. Factors associated with side effects were identified with logistic regression models for the full cohort and for each vaccine type adjusted for age, sex, race, education, rural residency, BMI, physical function (HAQ-II), COVID-19-specific stress (Likert scale), corticosteroid use, hierarchical DMARD group (reference: no DMARD), and diagnosis (reference: OA).
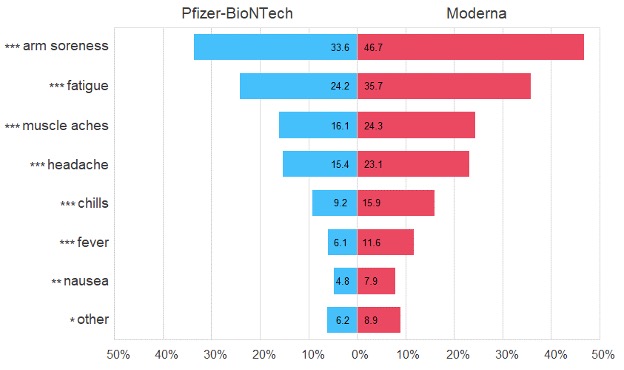
Results: Among 1825 vaccinated respondents, 876 (48%) reported experiencing side effects (Table 1). The most reported side effects included arm soreness (40%), fatigue (30%), muscle aches (20%), and headache (19%). The ranked frequency of each side effect did not differ by vaccine type, though incidence of each side effect was significantly higher among those who received the Moderna vaccine than in those who received the Pfizer-BioNTech vaccine (Figure 1). In the full cohort, younger age, female sex, higher education, full vaccination, and higher COVID-19-related stress were all associated with higher risk of experiencing side effects (Figure 2). Differences by age held true in both the Pfizer-BioNTech (OR 0.96 [0.94, 0.98]) and Moderna (0.97 [0.95, 0.99]) models. Differences by sex (OR 1.83 [1.04, 3.24]) and by COVID-19-related stress (1.5 [1.13, 2.00]) were specific to Moderna vaccine recipients, while differences by education (1.16 [1.04, 1.30]) were specific to Pfizer-BioNTech vaccine recipients.
Conclusion: Our findings of increased side effects to Moderna vaccines was consistent with the larger US population, though our side effect rates were comparatively lower and more similar to CDC surveillance1 after only the first mRNA vaccine even after accounting for our cohorts’ older age. A lack of reactogenicity with rheumatic disease, severity, and treatments may be reassuring for the vaccine hesitant, but additional investigation is needed to understand if these lower rates are due to a potentially reduced immune response.
1Chapin-Bardales et al. “Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines,” JAMA, 2021
To cite this abstract in AMA style:
Michaud K, Cornish A, Freifeld A, Katz P, Wipfler K. COVID-19 mRNA Vaccine Side Effects Among Individuals with Rheumatic Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/covid-19-mrna-vaccine-side-effects-among-individuals-with-rheumatic-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-mrna-vaccine-side-effects-among-individuals-with-rheumatic-disease/