Session Information
Date: Sunday, November 8, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Comorbidities
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: COVID-19 has overwhelmed the healthcare systems in New York City. Initial data from the Columbia Lupus Cohort suggests that 4% of patients with systemic lupus erythematosus (SLE) developed symptomatic COVID-19 infection, compared to the community transmission of 2% in New York City (Gartshteyn et al., 2020). There is no data about the impact of COVID on disease activity in SLE.
Methods: This study updates the earlier data with newly identified patients with SLE and COVID and describes post infection flares.
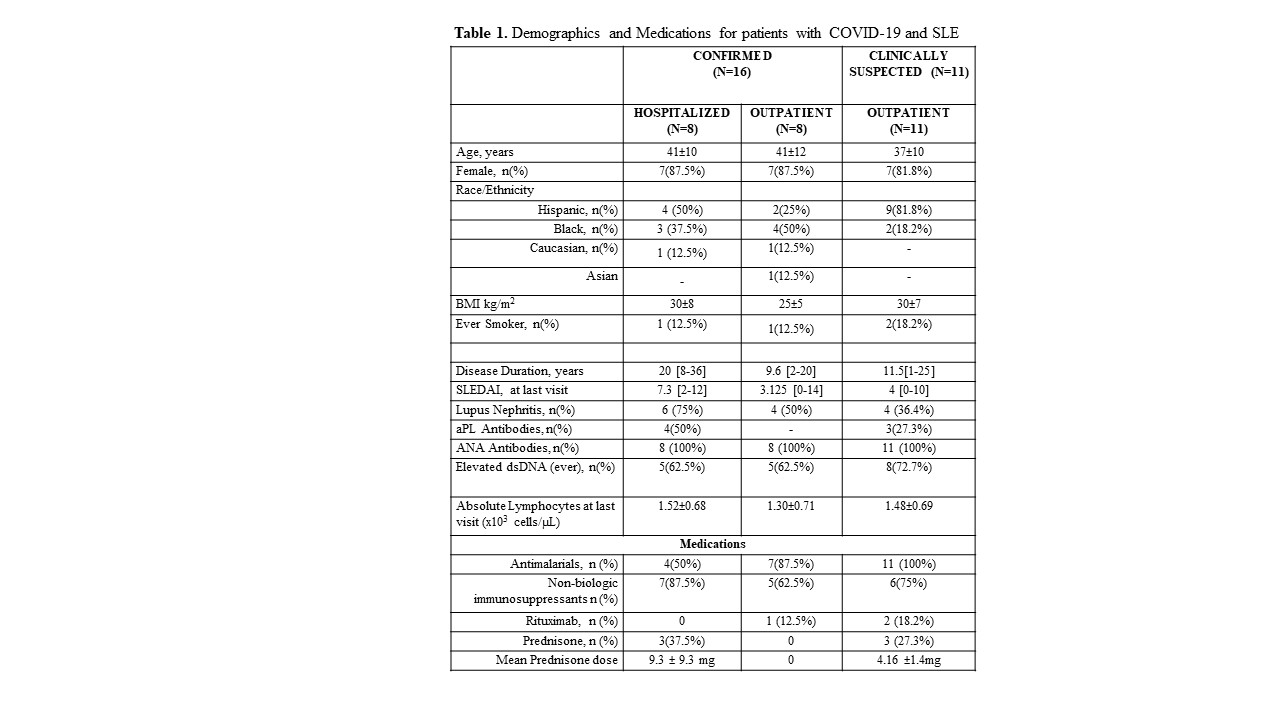
Results: From our cohort of 450 patients we identified and treated 27 SLE patients with COVID-19 infections (6%): 8 patients were hospitalized, 8 patients with confirmed infections were treated in an outpatient setting, and 11 patients with symptoms highly suggestive of COVID-19 were also treated as outpatient. Of the 16 confirmed cases, 12 patients had positive SARS-CoV-2 RT-PCR nasopharyngeal swabs and 4 patients had positive anti-SARS-CoV-2 antibodies.
77.8% of patients were female, mean age 40 ±10, 55.6% were Hispanic, 33.3% were Black, 3.7% were Asian, 7.4% were Caucasian, 81.5% of patients were taking antimalarials (hydroxychloroquine or chloroquine), 66.7% were taking non-biologic immunosuppressants, 11.1% had been treated with rituximab in the last 6 months, and 22.2% were either on prednisone or had taken prednisone within a week of their diagnosis. Their COVID-19 symptoms included fever (85.2%), myalgia (22.2%), cough (55.6%), shortness of breath (37%), chest pain (18.5%), and anosmia (7.4%).
Out of the 8 hospitalized patients 3 were critically ill with severe hypoxemic respiratory failure, one required mechanical ventilation. All received empiric antibiotics, 3 received high-dose IV methylprednisolone and tocilizumab. One non-critically ill patient required supplemental oxygen via nasal cannula. All hospitalized patients survived and were discharged (2 to acute rehab). Of the 19 SLE patients treated outpatient, 7 (36.8%) received azithromycin as treatment for COVID-19, 3 were prescribed aspirin for empiric thromboprophylaxis, and one patient not already on HCQ, received a 5 day course of HCQ. All had resolution of symptoms without further escalation of care. Hospitalized patients were less likely to be taking HCQ at the time of COVID diagnosis compared to those treated as outpatient (p=0.04). No other SLE treatment differences were identified between the groups.
Of the 27 patients, 6 (22.2%) experienced an SLE disease activity flare within 22 days post-COVID (range 3-49 days). Of the 6 flares, 5 were mild/moderate and 1 severe using the SELENA Flare Index (SFI) definitions. The flare symptoms included arthritis -5, alopecia -2, low C3/C4 -3, positive dsDNA -2, rash -1 and pleurisy -1.
Conclusion: SLE patients in our cohort experienced COVID infections at a rate similar to that of the general population. Our data suggest that SLE patients may experience disease flares following COVID-19 infections and HCQ may be protective of severe COVID.
To cite this abstract in AMA style:
Khalili L, Gartshteyn Y, Schmidt N, Kapoor T, Geraldino-Pardilla L, Askanase A. COVID-19 Infections May Increase the Risk of SLE Flares [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/covid-19-infections-may-increase-the-risk-of-sle-flares/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-infections-may-increase-the-risk-of-sle-flares/