Session Information
Date: Tuesday, November 12, 2019
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The course of systemic sclerosis-associated interstitial lung disease (SSc-ILD) is heterogeneous; some patients may experience rapid decline in lung function, while others have relatively stable disease. Large patient registries, such as The European Scleroderma Trials and Research (EUSTAR) database, can assist in mapping the trajectory of SSc-ILD and identifying patients at risk of progression.
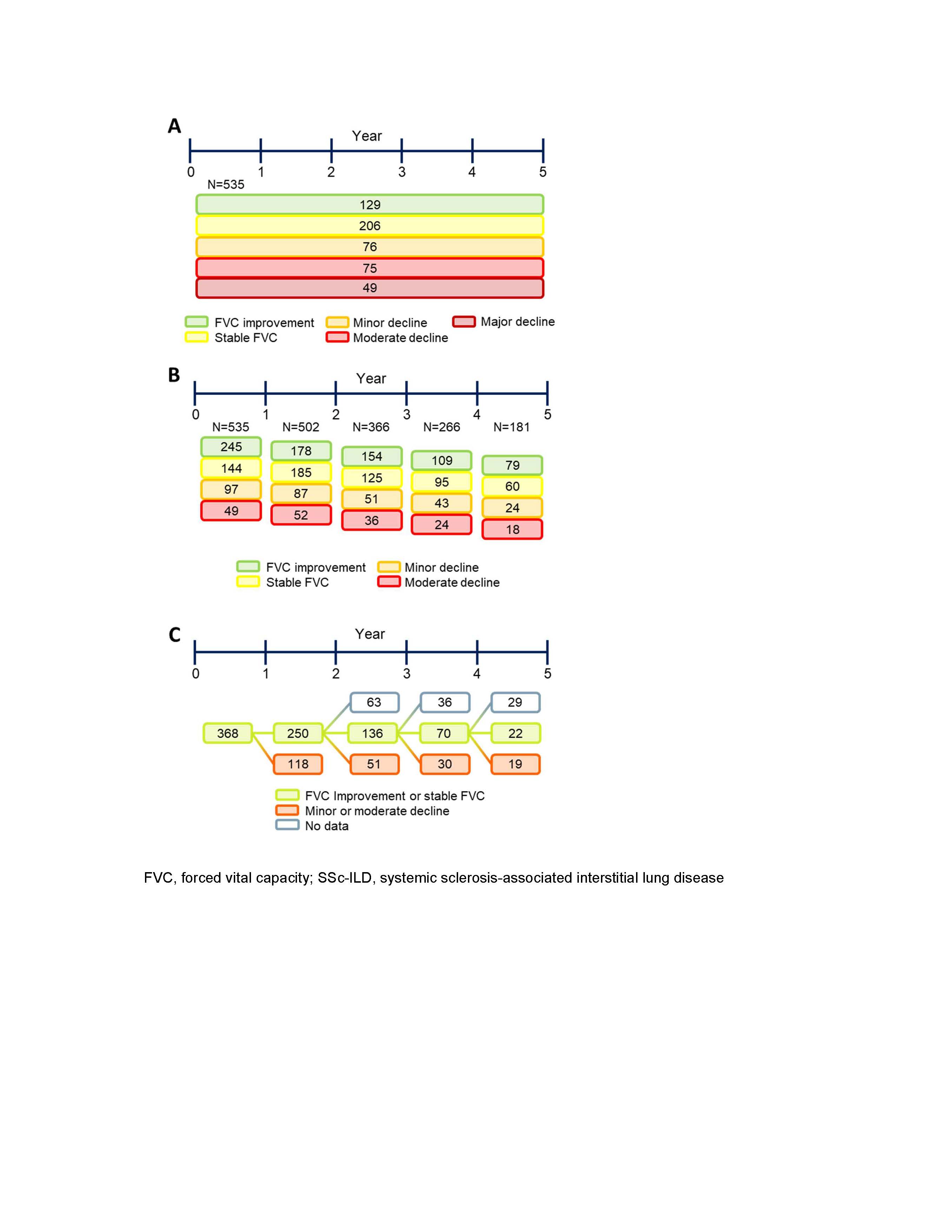
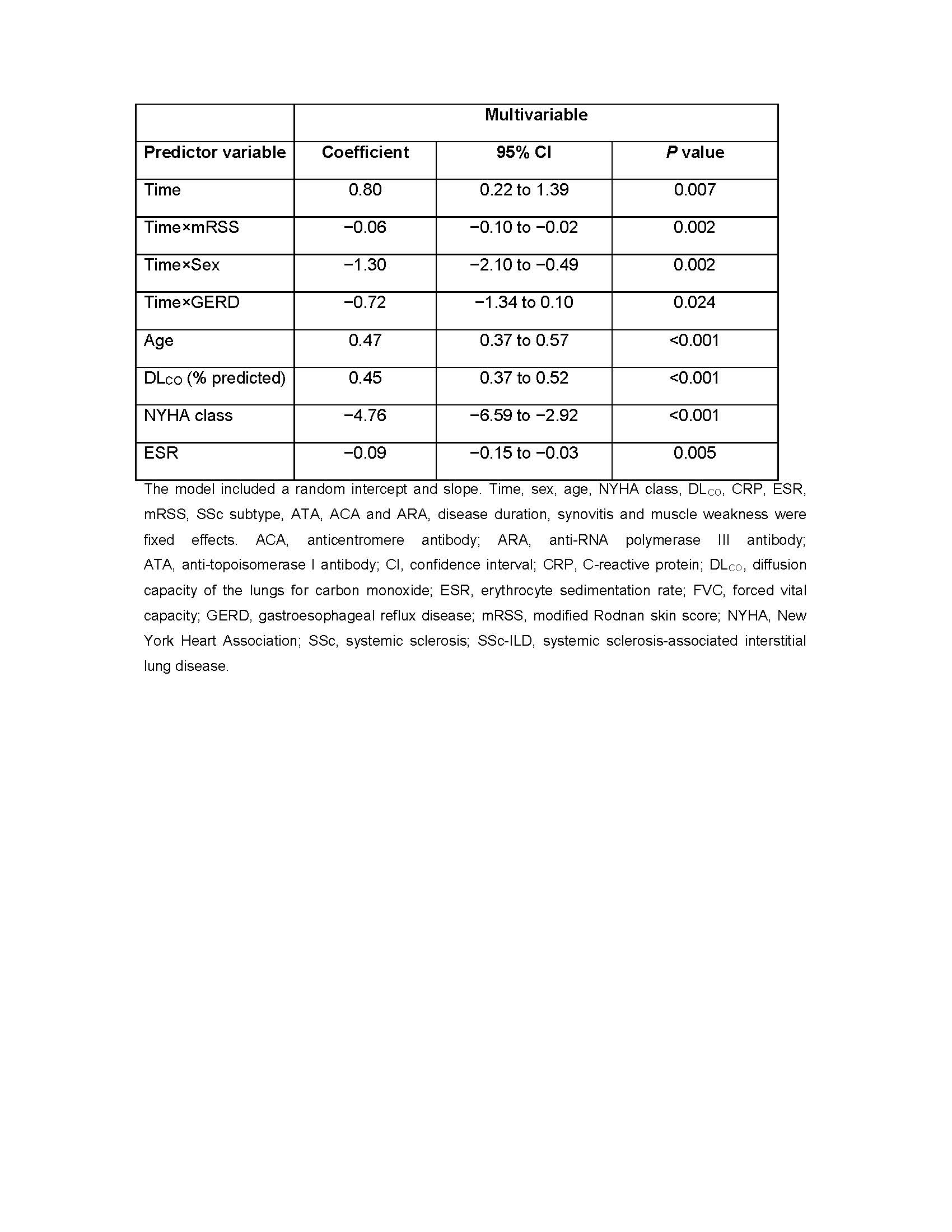
Methods: Patients with SSc-ILD by high-resolution computed tomography, according to American College of Rheumatology/European League Against Rheumatism criteria, who were registered in the EUSTAR database with lung function measurements at baseline and after 12±3 months, and known disease duration, were eligible. Patients had to have ≥3 serial annual forced vital capacity (FVC) measurements available. The overall FVC course was assessed using absolute changes in FVC (% predicted) from baseline to last available FVC measurement. Patients were divided into five subgroups: improved FVC (FVC increase of ≥5%); stable FVC (FVC decline or increase of < 5%); minor FVC decline (FVC decline of 5–10%); moderate FVC decline (FVC decline of >10–20%); and major FVC decline (FVC decline of >20%). Absolute FVC changes (% predicted) in individual patients in each 12-month interval during the 5-year follow-up period were then determined and defined as follows: FVC improvement (FVC increase of ≥5%); stable FVC (FVC decline or increase of < 5%); minor decline (FVC decline of 5–10%) and moderate decline (FVC decline of >10%). Candidate predictors of FVC decline were selected based on published reports and expert opinion and tested using linear mixed-effect regression analysis.
Results: Of 826 eligible patients with SSc-ILD, 535 (65%) had ≥3 serial annual FVC measurements available. Overall FVC course among these patients is shown in Figure 1A: over the mean 5-year follow-up period, 335 (63%) patients showed overall improved or stable FVC, while 200 (37%) patients experienced overall mild, moderate or major FVC decline. Among patients whose overall FVC course was improved or stable, 157 (47%) experienced one or more 12-month intervals in which their FVC declined by ≥5% (Table 1). In total during the observation period, 357 patients (67%) experienced one or more 12-month intervals with an FVC decline of ≥5% (Table 1; Figure 1B). 80% of patients who experienced a 12-month interval of FVC decline did not experience further decline in the following 12-month period. Male sex, gastroesophageal reflux disease, skin score, disease duration, age, diffusion capacity of the lungs for carbon monoxide, New York Heart Association class and erythrocyte sedimentation rate were baseline risk factors for FVC decline over a mean of 5 years (Table 2).
Conclusion: These results support close monitoring of patients with SSc-ILD, as most patients experienced at least one 12-month interval with FVC decline during their 5-year follow-up period. Moreover, intervals of FVC decline were seen in some patients who appeared stable overall. Importantly, the risk factors for FVC decline identified herein could help identify patients at risk of disease progression.
Funding: Boehringer Ingelheim International GmbH, Germany
To cite this abstract in AMA style:
Hoffmann-Vold A, Allanore Y, Alves M, Graf N, Airò P, Ananyeva L, Czirják L, Guiducci S, Hachulla E, Li M, Mihai C, Riemekasten G, Sfikakis P, Valentini G, Kowal-Bielecka O, Distler O. Course of Progressive Lung Fibrosis in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD) in the EUSTAR Database [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/course-of-progressive-lung-fibrosis-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-in-the-eustar-database/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/course-of-progressive-lung-fibrosis-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-in-the-eustar-database/