Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Sodium-glucose cotransporter-2 inhibitors (SGLT2i) lower serum urate levels and are associated with reduced risk of incident gout as well as recurrent flares [Annals IM 2023; JAMA IM 2024]. For some gout patients (e.g., without substantial hyperuricemia or radiographic damage or tophi), this could translate to reduced use of urate-lowering therapies (ULT) as well as gout flare medications such as NSAIDs or glucocorticoids, which can lead to cardiovascular-kidney-metabolic adverse effects, particularly among those with multi-morbidity and polypharmacy.
Our objective was to compare rates of initiation of allopurinol, as well as gout flare medication use (i.e., colchicine, NSAIDs, and glucocorticoids) among gout patients with type 2 diabetes (T2D) initiating SGLT2i versus dipeptidyl peptidase 4 inhibitors (DPP4i) or glucagon-like peptide-1 receptor agonists (GLP1-RA), two other second-line agents.
Methods: We followed the approach of Hernan and Robins to emulate two-arm randomized clinical trials (the target trials) that would randomize gout patients with T2D and no baseline use of ULT to initiate SGLT2i vs. GLP1-RA or DPP4i. We used administrative health data covering nearly all residents of British Columbia, Canada from Jan 2014 to June 2022, including all dispensed prescriptions, regardless of funder.
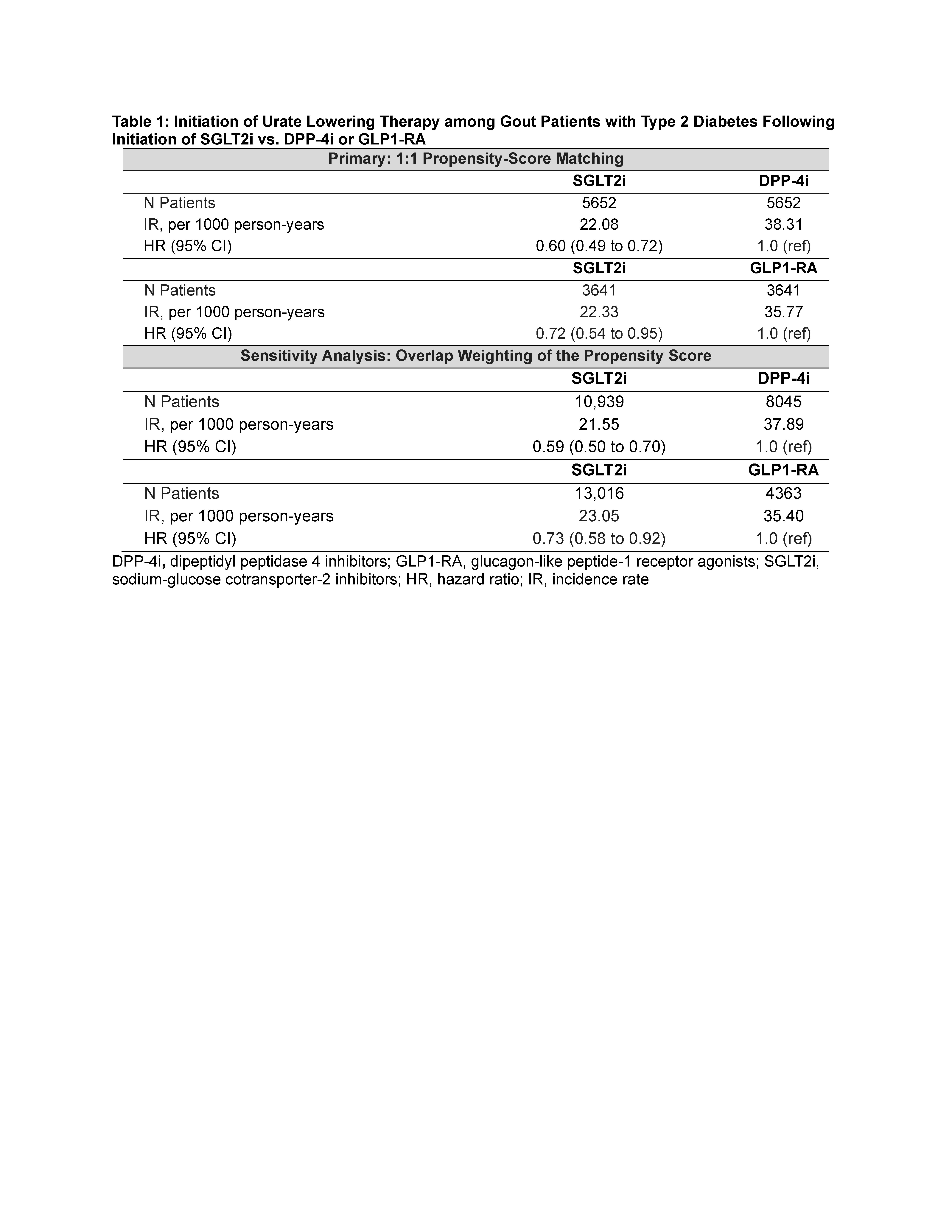
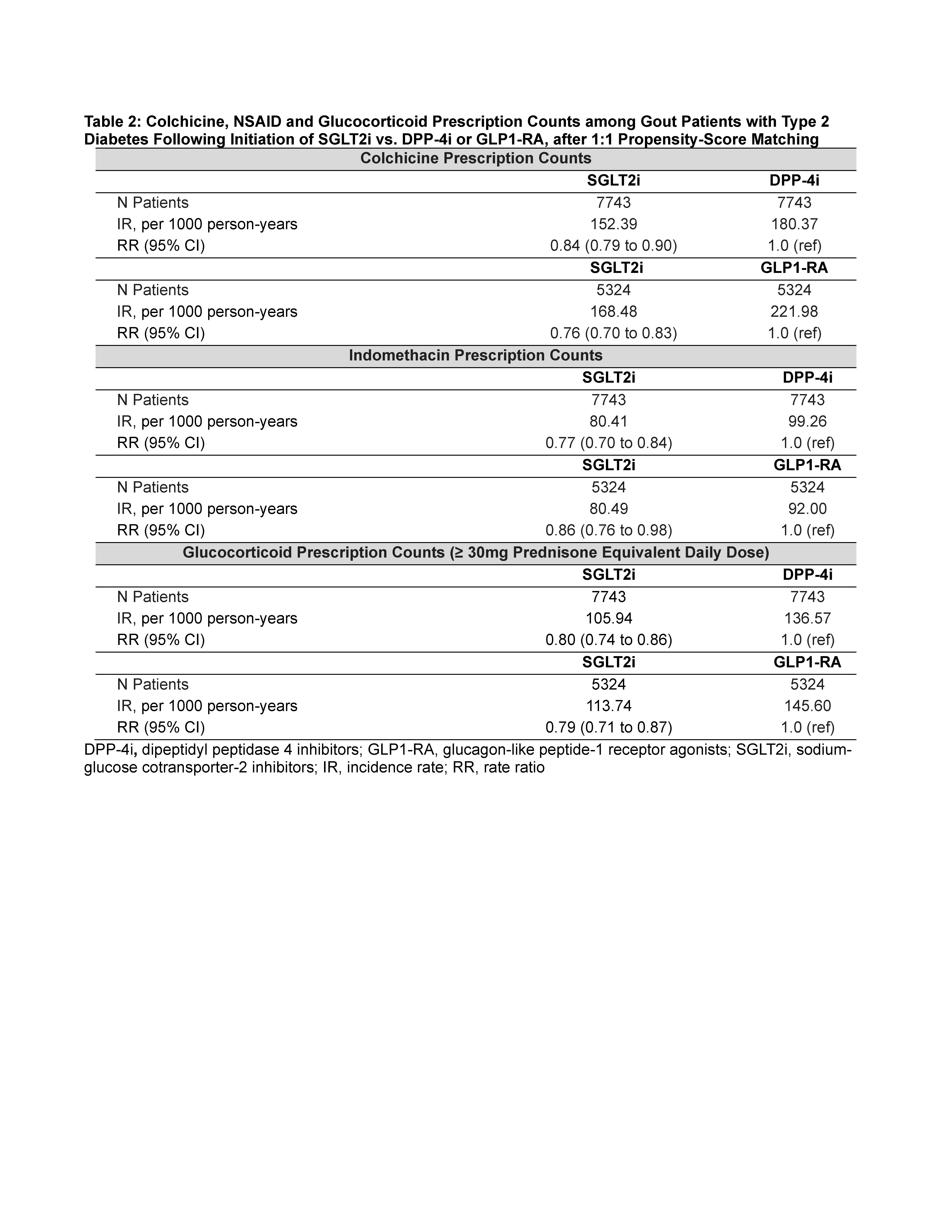
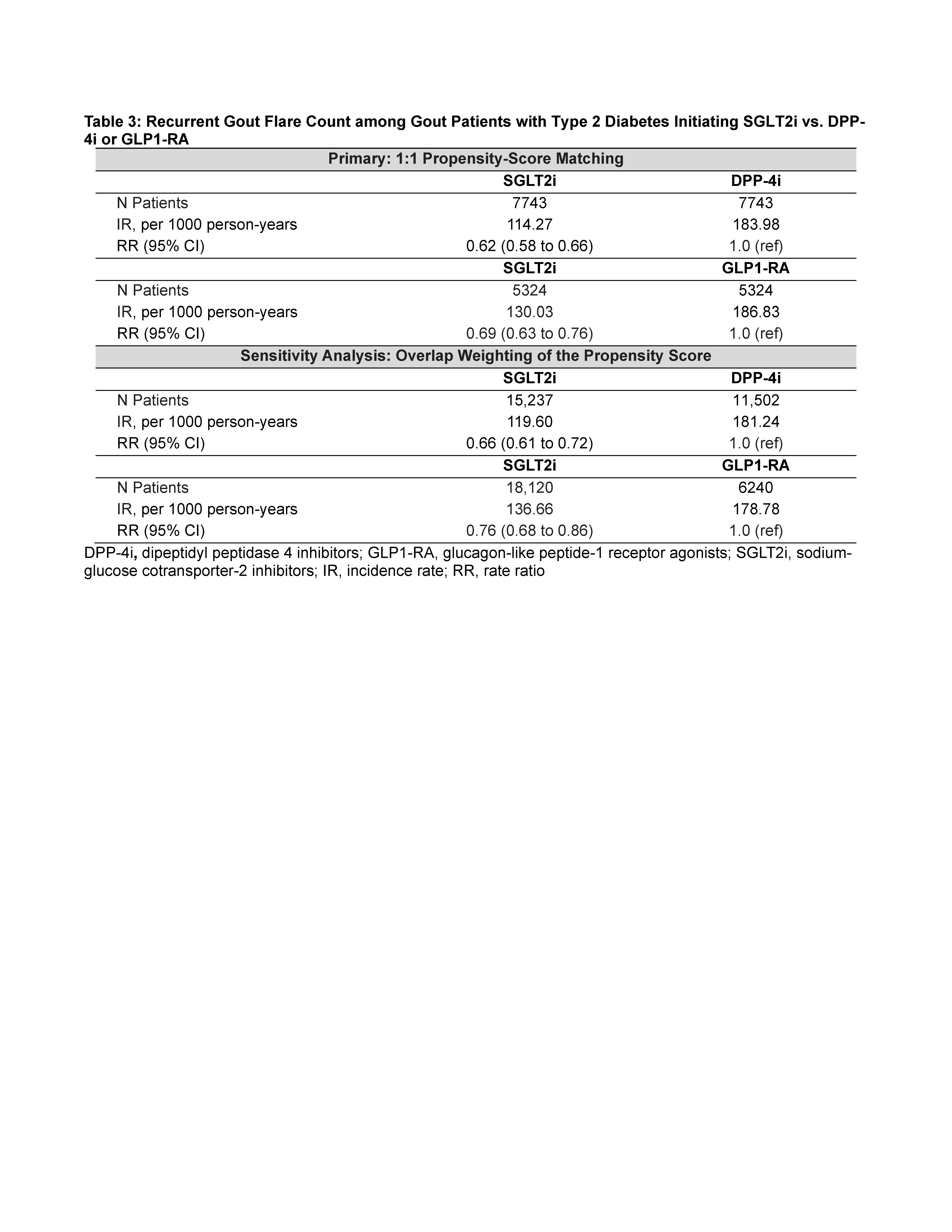
Primary outcome was new prescription of allopurinol. Secondary outcomes were number of prescriptions for colchicine, NSAIDs (indomethacin, most often used for gout flare) and glucocorticoids, and number of recurrent gout flares (defined by gout-primary outpatient and emergency visits, and hospitalizations). Cox proportional hazards and Poisson regressions were used with 1:1 propensity-score matching (primary) and overlap weighting (sensitivity analysis) to emulate randomization. The propensity score was constructed with >50 prognostic variables including comorbidities, medications, gout factors (duration, medication use, rheumatology encounters, baseline number of flares), socioeconomic status, and healthcare resource use.
Results: After matching, baseline characteristics of all treatment groups were well balanced (standardized mean differences < 0.1); the mean Charlson comorbidity score was 3.3. In our primary analysis, SGLT2i use was associated with decreased probability of allopurinol initiation, with hazard ratios (HR) of 0.60 (95% CI: 0.49, 0.72) for SGLT2i vs. DPP4i and 0.72 (0.54, 0.95) for SGLT2i vs GLP1-RA, (Table 1). Findings were similar when using overlap weighting of the propensity score.
SGLT2i was also associated with lower rates of colchicine, NSAID, and glucocorticoid dispensing (Table 2), and with lower rates of gout visits and recurrent flares (by 43% vs DPP4i and 27% vs GLP1-RA) (Table 3).
Conclusion: While many gout patients will continue to require conventional ULT, for those with modest hyperuricemia and infrequent flares, the serum urate-lowering benefits of SGLT2i may negate the need for additional ULT, by reducing the rate of recurrent flares. This could, in turn, reduce pill burden and exposure to the potentially harmful effects of NSAIDs and glucocorticoids, particularly for the highest risk patients with multi-morbidity and polypharmacy.
To cite this abstract in AMA style:
McCormick N, Yokose C, Lu L, Rai S, Challener G, Choi H. Could Initiation of Sodium Glucose Co-Transporter-2 Inhibitors Reduce the Need for Conventional Urate-Lowering Therapy and Flare Medications in Patients with Gout?Population-Based Target Trial Emulation Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/could-initiation-of-sodium-glucose-co-transporter-2-inhibitors-reduce-the-need-for-conventional-urate-lowering-therapy-and-flare-medications-in-patients-with-goutpopulation-based-target-trial-emulati/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/could-initiation-of-sodium-glucose-co-transporter-2-inhibitors-reduce-the-need-for-conventional-urate-lowering-therapy-and-flare-medications-in-patients-with-goutpopulation-based-target-trial-emulati/