Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Biologic Disease Modifying Anti-Rheumatic Drugs (bDMARDs) progressive tapering is a real opportunity in people living with rheumatoid arthritis (RA) having achieved remission both from the patient (reduction in the disease and drug-related burden) and the Society (cost alleviation) perspectives. The ToLEDo (Towards the Lowest Efficacious Dose) trial aimed to assess a disease activity-driven progressive tapering strategy of tocilizumab (TCZ) or abatacept (ABA) compared to their maintenance at full dose in RA patients in sustained remission. Non-inferiority (NI) was not demonstrated in terms of disease activity nor relapses, major relapses, radiographic progression. The aim of this secondary analysis was to assess the cost-utility of the spacing strategy (S-arm) in the ToLEDo trial compared to full dose maintenance (M-arm).
Methods: ToLEDo is a multicenter 2-year NI randomized open-label controlled trial, which enrolled 228 patients (113 in the S-arm and 115 in the M-arm). A cost-utility analysis was conducted on the per protocol population. In each arm, health benefits were estimated every 6 months by Short Form Health Survey (SF-6D) and EuroQoL (EQ-5D)-derived utility measurements. Cost elicitation integrated health resource use including bDMARD costs (direct cost) as well as productivity loss (indirect cost).The incremental cost-utility ratios (ICUR) were calculated by dividing the difference of costs between S-arm and M-arm by the difference of utilities between the 2 arms. 95% confidence interval (95%CI) were calculated by bootstrap (20,000 iterations). The incremental net benefit (INB) was calculated for willingness to pay (WTP) values ranging from 0 to 150,000€. The analyses were replicated using SF-6D (primary analysis) or EQ-5D, and in ABA and TCZ subgroups. Acceptability analyses as well as stochastic sensitivity analyses (simulating costs and utilities using MCMC algorithms) were also performed.
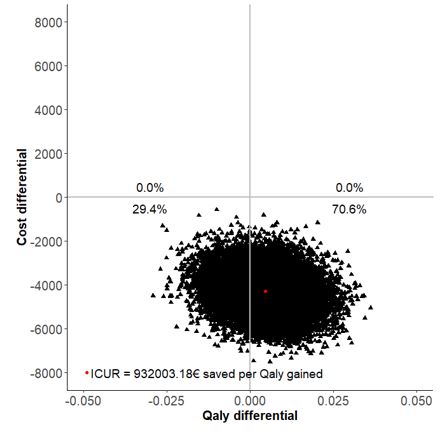
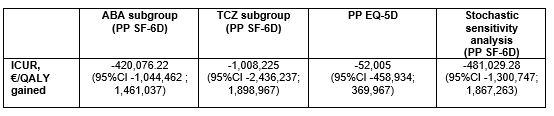
Results: Overall, 178 patients were included (82 in S-arm, 96 in M-arm) in the per protocol analysis. At the end of the follow-up in the S-arm, 15.0% of patients discontinued their biologic, 48.7% spaced the injections, and 36.3% remained at the standard dose. The difference in terms of two-years utility gains between S-arm and M-arm was 0.004 (95%CI -0.012, 0.021) with SF-6D. The difference of total costs between S-arm and M-arm was -4,275 € (95%CI -5,955 to -2,542). The estimated ICUR of the spacing strategy over the maintenance at full dose was €932,003 saved per QALY (95% CI -7,534,788 to 6,720,372) with SF-6D. The INB was 4,734.6€ for a WTP of 100,000€. With a willingness to accept of 0 €/QALY lost, the probability to be cost-effective for the spacing strategy was 70.6% (Figure 1). The results were consistent when using EQ-5D-derived utilities, in ABA and TCZ subgroups, as well as in the stochastic sensitivity analyses (Table 1).
Conclusion: Although the ToLEDo trial did not demonstrate non-inferiority, the tested disease activity-driven tapering strategy was not associated with health loss in terms of utilities and incurred for substantial cost savings, making this strategy potentially dominant.
To cite this abstract in AMA style:
Kedra J, Granger B, El Houari L, Tubach F, Fautrel B. Cost-utility of a Progressive Spacing of Tocilizumab or Abatacept in Patients with Rheumatoid Arthritis in Sustained Remission: A Medico-economic Analysis of the Towards the Lowest Efficacious Dose Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cost-utility-of-a-progressive-spacing-of-tocilizumab-or-abatacept-in-patients-with-rheumatoid-arthritis-in-sustained-remission-a-medico-economic-analysis-of-the-towards-the-lowest-efficacious-dose-tr/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cost-utility-of-a-progressive-spacing-of-tocilizumab-or-abatacept-in-patients-with-rheumatoid-arthritis-in-sustained-remission-a-medico-economic-analysis-of-the-towards-the-lowest-efficacious-dose-tr/