Session Information
Date: Saturday, November 12, 2022
Title: Health Services Research Poster I: Lupus, RA, Spondyloarthritis and More
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
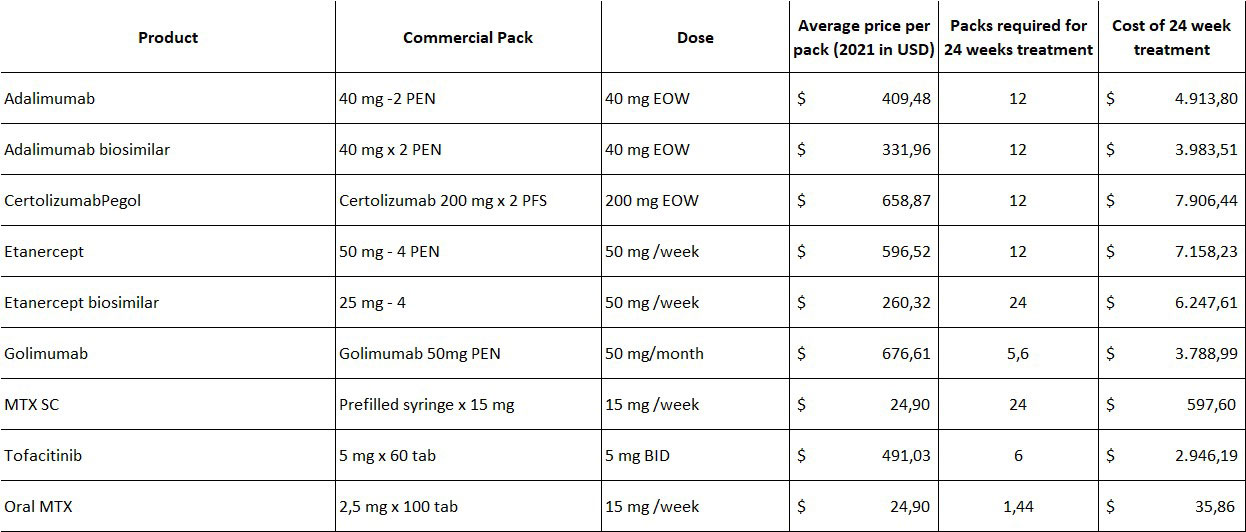
Background/Purpose: The Colombian healthcare system is under economic pressure like many other systems in the world. In that sense, treatment choice involves cost in addition to the clinical benefits. To provide a fair comparison of Rheumatoid Arthritis (RA) treatments used to treat patients failing first line therapy in Colombia, a cost-per-responder analysis can objectively estimate the impact of treatment costs on the healthcare system.
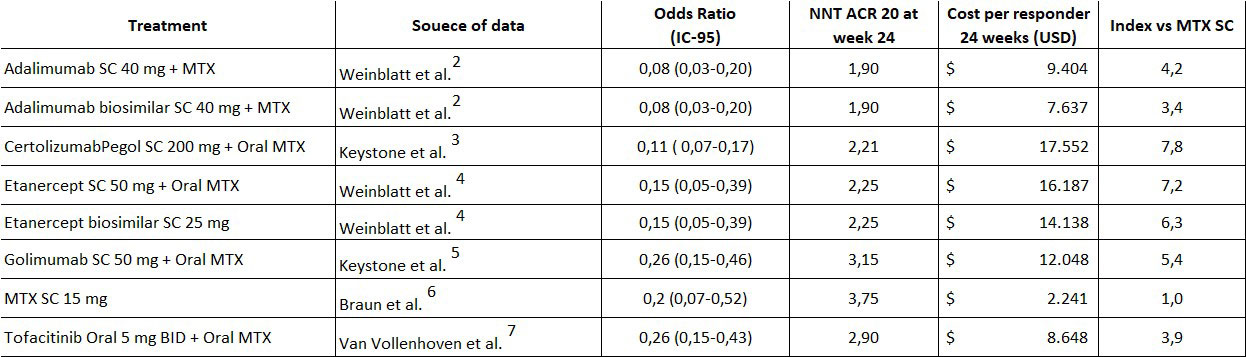
Methods: A calculation of the number needed to treat (NNT) was developed taking the second line studies after failure to first line DMARD for anti-TNF, subcutaneous methotrexate, and tofacitinib with ACR20 at 24 weeks as the comparison outcome for all clinical trials selected. The costs for each product were calculated by using the average price registered in the National Price Database (SISMED) and converted into US dollars with the official rate registered by the national bank on June 8th, 2022. The average doses to calculate the monthly and 24-week treatment costs were based on the prescribing information for each product included in the study (Table 1). As there is no 24-week study with ACR20 outcome for adalimumab and etanercept biosimilars, the data used for the calculation was the information provided in the originator studies. Finally, the cost per responder was calculated by multiplying the monthly cost by the length of treatment (24 weeks) with the addition of MTX cost with an average dose of 15 mg/week as defined in the selected clinical trials.
Results: As it is shown in Table 2, the lower NNT was achieved with adalimumab for ACR20 at 24 weeks while the highest was obtained with SC MTX. On the other hand, in the Cost per responder analysis, the most favorable result was obtained with SC MTX being 71% below the cost of adalimumab biosimilar which resulted in a 3,4 times higher index.
Conclusion: The cost-per-responder analysis supports the use of SC MTX as a strategy to optimize treatment costs for RA patients failing first line DMARDs (including oral MTX) and may generate important savings for the Healthcare System.
To cite this abstract in AMA style:
Cardozo M. Cost Per Responder in RA Patients Failing First Line Treatment [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/cost-per-responder-in-ra-patients-failing-first-line-treatment/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cost-per-responder-in-ra-patients-failing-first-line-treatment/