Session Information
Date: Wednesday, November 13, 2019
Title: 6W017: Health Services Research II: Health Economics (2888–2893)
Session Type: ACR/ARP Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Knee OA is a disabling condition affecting over 14 million adults in the US. NSAIDs provide only short-term pain relief, creating the need for additional pain management. Growing evidence indicates that chronic knee OA pain is associated with centralized pain pathways. Based on these considerations, ACR and OARSI recommend or conditionally recommend the use of duloxetine. The cost-effectiveness of generic duloxetine in the US has not been assessed in knee OA.
Methods: We used the Osteoarthritis Policy Model, a validated widely published microsimulation of knee OA, to evaluate the cost-effectiveness of adding duloxetine to usual care (UC) after NSAIDs fail to maintain pain relief for knee OA patients. The UC included intra-articular injections, tramadol, oxycodone, and total knee replacement (TKR). We generated the cohort with the demographic and clinical characteristics (age, BMI, and radiographic severity measured as Kellgren-Lawrence (KL) grade) of knee OA patients who failed NSAID treatment. We then conducted the evaluation of four different cohorts stratified by pain severity (starting WOMAC Pain (0-100, 100 is worst) scores of 25, 35, 45, and 55) and continued treatment with regimens that either did or did not contain duloxetine. We followed subjects until death, recording quality-adjusted life years (QALYs) accrued, total lifetime medical costs, likelihood of using opioids and/or TKR. We used national databases and previously published data to estimate the probability of KL progression, quality of life utilities (stratified by pain, number of comorbidities, age, and BMI), treatment efficacy, direct medical costs of knee OA treatments ($800 for NSAIDs and tramadol, $1,100 for oxycodone, and $1,200 for duloxetine), cost and risk of complications. We discounted costs and QALYs at 3%/year and used a healthcare perspective. We assessed cost-effectiveness by evaluating incremental cost-effectiveness ratios (ICERs) calculated as the ratio of change in costs to change in QALYs for two competing strategies at a Willingness-to-Pay (WTP) threshold of $100,000/QALY.
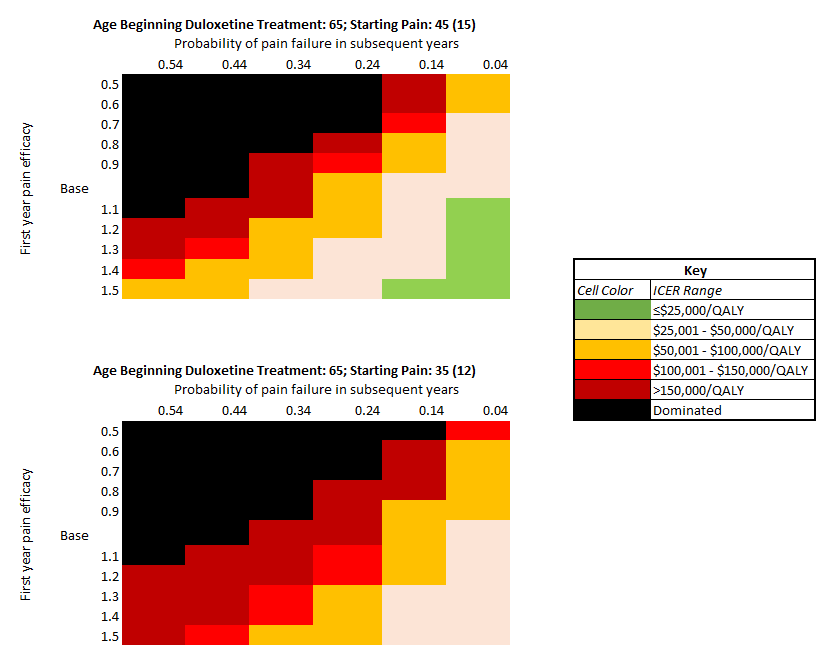
Results: Adding duloxetine in addition to UC treatments in OA patients with high pain (45 (15) WOMAC points) resulted in adding 2 QALYS per 100 persons taking duloxetine and increased direct lifetime medical costs by $1,500 per person with resulting ICER of $81,000/QALY. Knee OA patients spent on average 2.25 years on duloxetine. Based on the age of starting duloxetine (56 vs. 75 years), duloxetine reduced the risk of opioid use by 7.2%-15.6% and reduced the use of TKR by 6.8% -12.5%. Duloxetine was not cost-effective (at $100,000 WTP) in those with lower pain (25 or 35 WOMAC points) with resulting ICERs of $580,000 and $148,000/QALY respectively. 2-way sensitivity analyses of pain efficacy and failure found the cost-effectiveness of duloxetine sensitive to both regardless of starting pain (Figure 1).
Conclusion: The addition of duloxetine to the UC for knee OA after failure of NSAIDs is cost-effective for those with high pain and may lead to appreciable reduction in risk of opioid use and utilization of TKR. Use of duloxetine in OA patients with mild pain is not justified based on cost-effectiveness.
To cite this abstract in AMA style:
Sullivan J, Katz J, Losina E. Cost-effectiveness of Duloxetine for Knee OA Patients Whose Pain Can’t Be Controlled by NSAIDs [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/cost-effectiveness-of-duloxetine-for-knee-oa-patients-whose-pain-cant-be-controlled-by-nsaids/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cost-effectiveness-of-duloxetine-for-knee-oa-patients-whose-pain-cant-be-controlled-by-nsaids/