Session Information
Date: Monday, November 6, 2017
Title: Health Services Research Poster II: Osteoarthritis and Rheumatoid Arthritis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Adalimumab (ADA) induces and maintains clinical remission in patients with rheumatoid arthritis (RA) and Crohn’s disease (CD) but is an expensive drug. Drug-level and antidrug antibody testing has the potential to facilitate personalization of dosing, particularly the use of less frequent dosing of ADA (de-escalation). The cost-effectiveness of drug-level guided dose de-escalation of therapy is unknown.
Methods: We evaluated the effectiveness (in quality adjusted life years [QALYs]) and cost-effectiveness of a drug-level guided dose-reduction strategy for ADA compared with standard dosing in patients with CD who are in clinical remission, from a health system perspective using a 4-year horizon. We chose CD rather than RA because drug-level guided dose de-escalation studies have not been conducted in RA. We developed a discrete-time Markov simulation model for the analysis, using inputs from published randomized controlled trials and observational studies. The intervention studied was reduction in frequency of ADA dosing from 40mg every other week (EOW) to 40mg every three weeks (ETW) among patients with sustained clinical remission and therapeutic ADA trough levels. Patients who lost clinical remission would undergo drug-level testing. A switch to an alternate biologic was indicated with positive antidrug antibodies or drug resistance (adequate levels despite active symptoms); otherwise the dose was re-escalated back to 40mg EOW. Standard dosing (control arm) involved continuation of 40mg EOW dosing and switch to an alternate biologic on loss of clinical remission.
Results: Among patients with CD in remission on standard dose ADA, reductions in dose yielded similar QALYs but lower costs compared with continuing standard dosing strategy. The difference between the standard and dose-reduction strategies ranged from 0.00 to 0.06 QALYs across the range of probabilities tested (with standard strategy being marginally more effective), but the intervention resulted in cost-savings in every circumstance. In sensitivity analyses, the dose-reduction strategy was the dominant option (less costly and more effective) when ≥87% of patients who were controlled on standard dosing continued to be in remission under the dose-reduction strategy. The findings were most sensitive to the probability of formation of antidrug antibodies after dose reduction and the cost of ADA.
Conclusion: Based on lower costs and similar overall efficacy, dose reduction may be a cost-saving alternative among patients with CD who are in sustained remission with therapeutic ADA levels. These findings suggest that further research to explore drug-level guided tapering in RA may also be warranted. Given the small difference we observed in incremental effectiveness, our proposed strategy may prove to be dominating if future studies also demonstrate that reduced dosing improves the safety of ADA.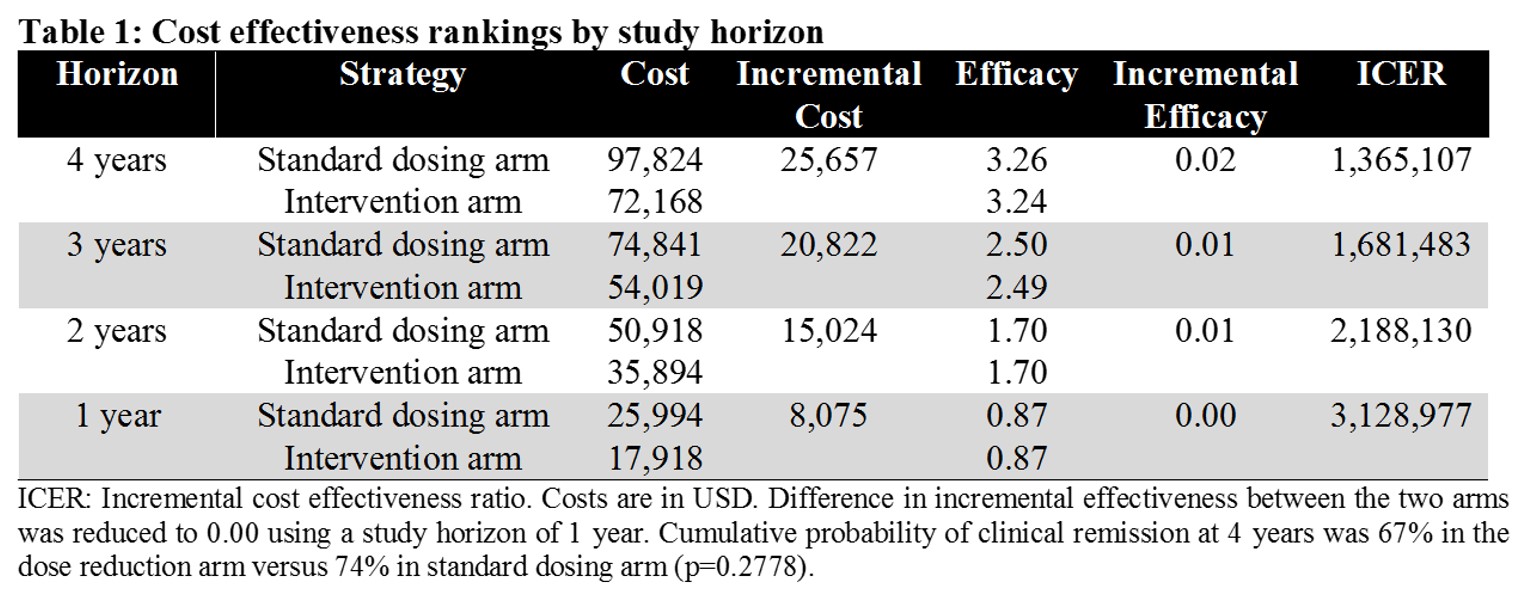
To cite this abstract in AMA style:
Izadi Z, Schmajuk G, Gianfrancesco M, Trupin L, Jafri K, Yazdany J, Kazi D. Cost-Effectiveness of Drug-Level Guided Adalimumab Dosing [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/cost-effectiveness-of-drug-level-guided-adalimumab-dosing/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cost-effectiveness-of-drug-level-guided-adalimumab-dosing/
