Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Biologic Disease Modifying Anti-Rheumatic Drugs (bDMARDs) progressive tapering is a real opportunity in people living with rheumatoid arthritis (RA) having achieved remission both from the patient (reduction in the disease and drug-related burden) and the Society (cost alleviation) perspectives. The ToLEDo (Towards the Lowest Efficacious Dose) trial aimed to assess a disease activity-driven progressive tapering strategy of tocilizumab (TCZ) or abatacept (ABA) compared to their maintenance at full dose in RA patients in sustained remission. Non-inferiority (NI) was not demonstrated in terms of disease activity (primary endpoint) or relapses, major relapses, radiographic progression (secondary endpoints).
The aim of this secondary analysis was to assess the cost-effectiveness of the spacing strategy (S-arm) in the ToLEDo trial compared to full dose maintenance (M-arm).
Methods: The ToLEDo trial was a multicenter 2-year NI randomized open-label controlled trial, which enrolled 228 patients (113 in the S-arm and 115 in the M-arm). A cost-utility analysis was conducted on the per protocol population of ToLEDo.
In each arm, health benefits were estimated every 6 months by Short Form Health Survey (SF-6D) and EuroQoL (EQ-5D)-derived utility measurements. Cost elicitation integrated health resource use including bDMARD costs (direct cost) as well as productivity loss (indirect cost) using the friction cost method. The incremental cost-utility ratios (ICUR) were calculated by dividing the difference of costs between S-arm and M-arm by the difference of utilities between the 2 arms. 95% confidence interval (95%CI) were calculated by bootstrap (20,000 iterations). Acceptability analyses were also performed.
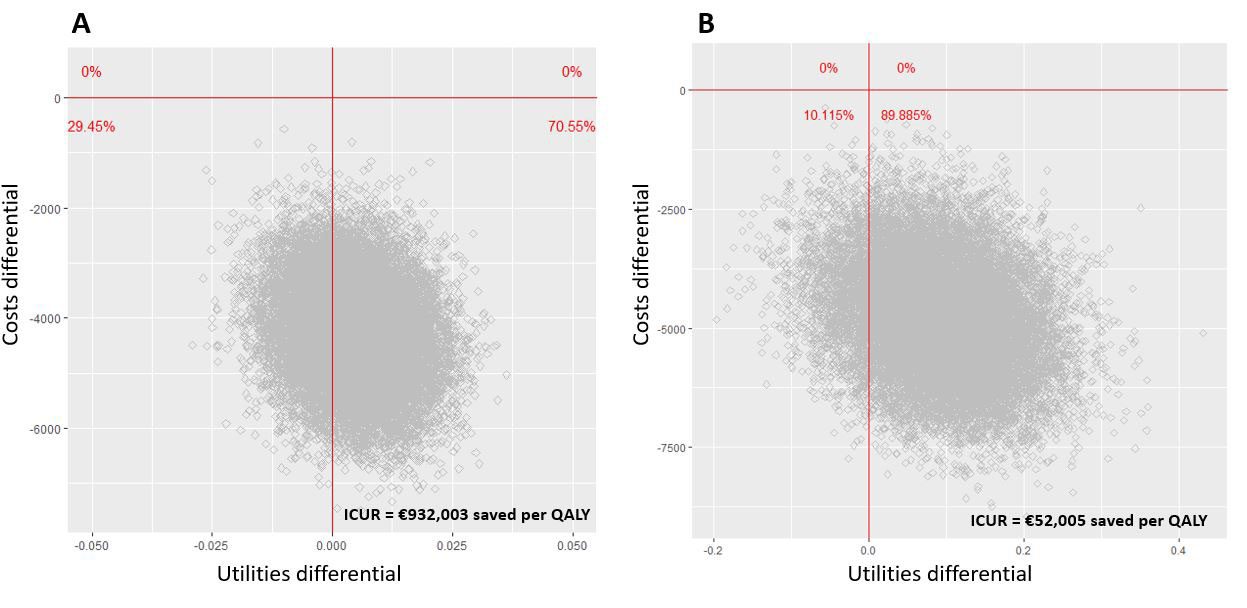
Results: Overall, 178 patients were included (82 in S-arm, 96 in M-arm) in the per protocol analysis. At the end of the follow-up in the S-arm, 15.0% of patients discontinued their biologic, 48.7% spaced the injections, and 36.3% remained at the standard dose. Two-year utility gains were 1.156 (95%CI 1.143, 1.170) and 1.152 (95%CI 1.142, 1.162) QALY in the S-arm and M-arm respectively with SF-6D, and 1.541 (95%CI 1.429, 1.638) and 1.449 (95%CI 1.351, 1.541) respectively with EQ-5D; the difference in terms of utility gains between S-arm and M-arm was therefore 0.004 (95%CI -0.012, 0.021) with SF-6D and 0.09 (95%CI -0.05, 0.23) with EQ-5D. The difference of total costs between S-arm and M-arm was -4,275 € (95%CI -5,955 to -2,542) with SF-6D and -4,781 € (95%CI -6,905 to -2,507) with EQ-5D. The estimated ICUR of the spacing strategy over the maintenance at full dose was €932,003 saved per QALY (95% CI -7,534,788 to 6,720,372) with SF-6D and €52,005 saved per QALY (95%CI -458,934 to 369,967) with EQ-5D. With a willingness to accept of 0 €/QALY lost, the probability to be cost-effective for the spacing strategy ranged between 70.6% and 89.9% (Figure 1).
Conclusion: Despite the fact that the ToLEDo trial did not demonstrate its non-inferiority, the tested disease activity-driven tapering strategy was not associated with health loss in terms of utilities and incurred for substantial cost savings, making this strategy potentially dominant.
To cite this abstract in AMA style:
KEDRA J, El Houari L, Tubach F, granger B, Fautrel B. Cost-Effectiveness of a Progressive Spacing of Tocilizumab or Abatacept in Patients with Rheumatoid Arthritis in Sustained Remission: A Medico-Economic Analysis of the ToLEDo Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/cost-effectiveness-of-a-progressive-spacing-of-tocilizumab-or-abatacept-in-patients-with-rheumatoid-arthritis-in-sustained-remission-a-medico-economic-analysis-of-the-toledo-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cost-effectiveness-of-a-progressive-spacing-of-tocilizumab-or-abatacept-in-patients-with-rheumatoid-arthritis-in-sustained-remission-a-medico-economic-analysis-of-the-toledo-trial/