Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid Arthritis (RA)patients who switch from oral to subcutaneous methotrexate (MTX) may experience better response to treatment due to increased bioavailability, enhanced tolerability, increased adherence, fewer side effects and better clinical response rates. However, the economic impact of such a switch is unknown. The study objective was to evaluate the incremental cost associated with subcutaneous (Rasuvo®) vs. oral MTX treatment in RA patients.
Methods: A comparative effectiveness study was conducted in adult (18+ years) RA patients on MTX treatment, identified from the Kaiser Permanente Southern California health plan member population. We employed a prospective cohort study design with 1:1 matching of oral vs. Rasuvo® users. After obtaining informed consent, patients completed either a web based or mailed paper survey which recorded their baseline disease activity scores using the MDHAQ’s RAPID3 measure. We analyzed direct medical expenditure associated with medical office visits (routine and urgent care), hospital outpatient and ED visits, inpatient and observation stays, pharmacy utilization, laboratory, and radiology utilization during the one-year following the index prescription fill for MTX. To mitigate potential bias due to non-random treatment assignment, we used 2-stage residual inclusion instrumental variables (2SRI) techniques to evaluate the incremental effect of oral MTX vs Rasuvo® on total all cause expenditure as well as subgroups of utilization. Depending on the degree of zero mass, either one part or two part generalized linear model with log link and gamma family distribution with 2SRI correction were specified while confidence intervals were based on 1000 bootstrapped repetitions of these outcome models. Each utilization model adjusted for baseline disease activity (based on MDHAQ survey), duration of RA (early within 2-years of diagnosis); duration of MTX use; age, female sex, white race, Hispanic ethnicity, smoking status, body mass index over 30, income category and Charlson comorbidity index.
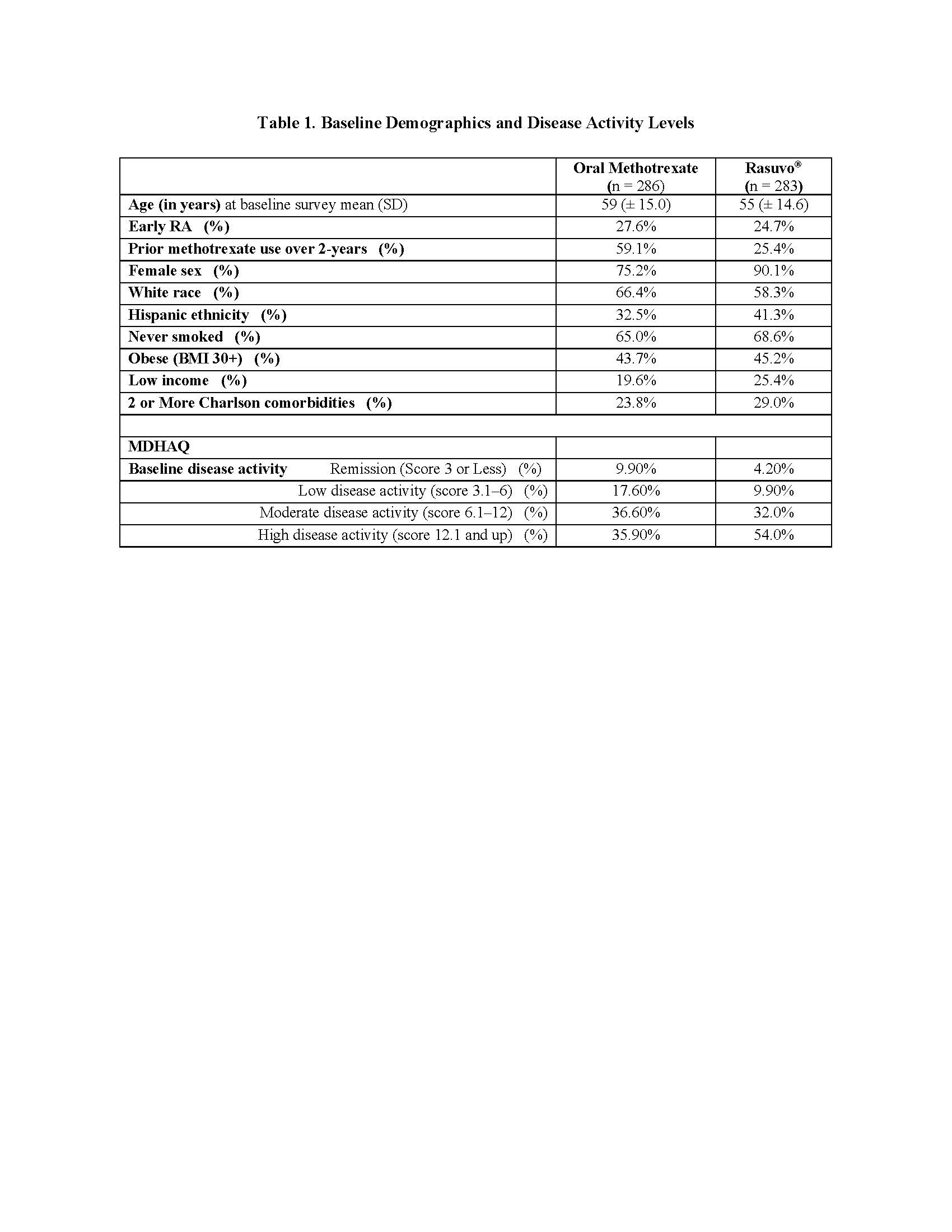
Results: The baseline self-report survey completion rate was 20% (569/2885; oral methotrexate = 286 and Rasuvo® = 283). The mean age of the sample was 57 (±15) years with the majority (83%) of female (Table 1). Rasuvo® arm included a higher proportion of patients with multiple comorbid conditions (29% vs 24%) and who also had higher proportion reporting high disease activity (54% vs 36%) (Table 1). Rasuvo® use was associated with significantly higher all cause total expenditure $4,704 (95% CI $1,758 to $7,650) which was predominantly a result of higher costs associated with all cause pharmacy utilization $4,223 (95% CI $1,460 to $6,986) (Table 2). None of the other subgroups of utilization had statistically significant differences.
Conclusion: In this prospective comparative effectiveness study, patients on subcutaneous methotrexate were associated with higher total cost which was driven by increased pharmacy expenditure.
To cite this abstract in AMA style:
Kawatkar A, Yi D, Estrada E, Portugal C. Cost Analysis of Subcutaneous Methotrexate Compared to Oral Methotrexate Treatment for Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cost-analysis-of-subcutaneous-methotrexate-compared-to-oral-methotrexate-treatment-for-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cost-analysis-of-subcutaneous-methotrexate-compared-to-oral-methotrexate-treatment-for-rheumatoid-arthritis/