Session Information
Date: Friday, November 6, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Clinical Manifestations
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: SLE is managed by variable combinations of five drug classes: antimalarials, biologics, corticosteroids, non-steroidal anti-inflammatory agents, and immunosuppressants. Opioids are commonly prescribed to SLE patients despite not being effective for the management of long-term musculoskeletal pain.1 The aim was to describe corticosteroid and opioid use among SLE patients in the United States, and the impact of belimumab initiation on prescribing patterns.
Methods: This retrospective study used MarketScan administrative claims databases to select insured adults, age >18, with a diagnosis (International Classification of Diseases (ICD)-9/10 710.0 & M32) of SLE between 1/1/2012 and 5/31/2018 (earliest SLE diagnosis = index date). Patients were followed from index through the earliest of health plan disenrollment or 5/31/2019 (minimum of 12 months). Corticosteroid use was measured in the 12 months following SLE index date. Average daily dose of oral corticosteroids in prednisone equivalents was measured for 12 months after corticosteroid initiation. Opioid use was measured overall, and by strength and length of treatment (chronic use defined as >90 days of supply). Oral corticosteroid and opioid use were compared in the 6 months before and after initiation of belimumab.
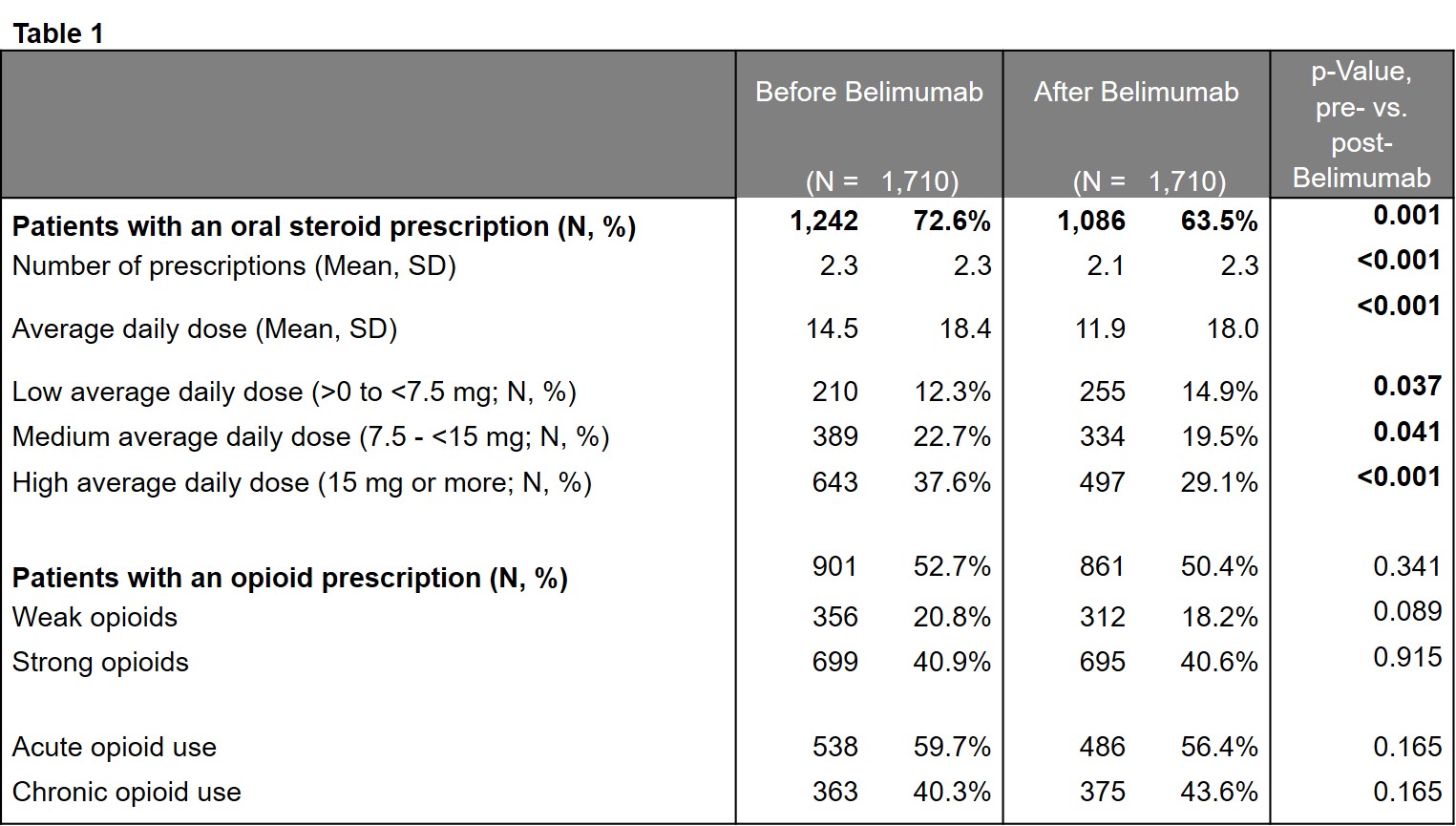
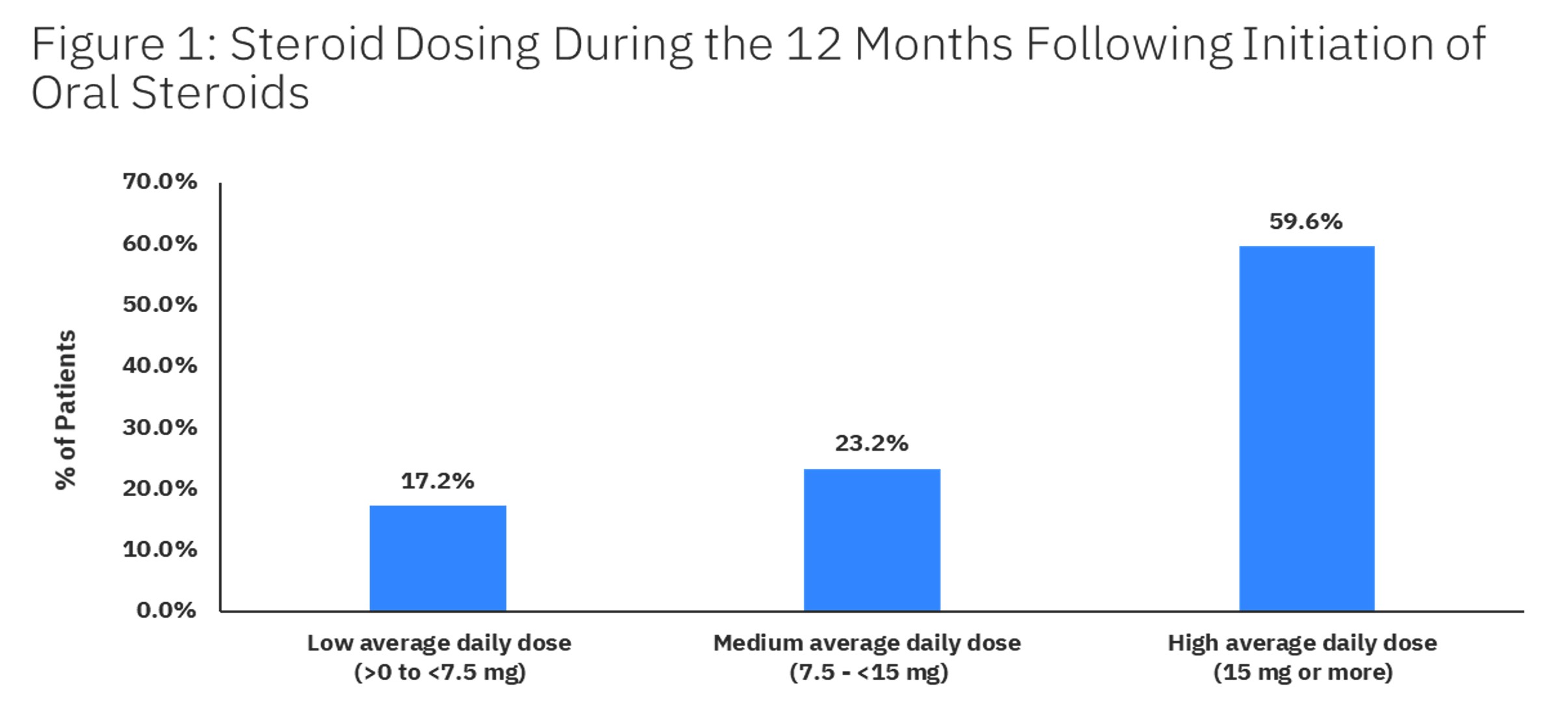
Results: Of 49, 413 SLE patients eligible for analysis, mean [SD] age was 50.1 [14.0] years, 90.2% were female, and average follow-up was 3.6 [1.9] years. 89.8% of patients received any SLE treatment and 68.5% received corticosteroids. The average number of corticosteroid prescriptions was 4.6 [4.1] during 12 months of follow-up. 52.6% of patients had ≥1 claim for an opioid prescription in the 12 months after SLE index and 34.6% were identified as having chronic opioid treatment. Among patients with oral corticosteroid treatment and 12 months of study enrollment post-corticosteroid initiation, the average daily dose for oral corticosteroids was 19.4 [14.2] mg and 59.6% had a high average daily dose of >15 mg (Figure 1). Among 1,710 patients with belimumab treatment and 6 months of study enrollment after the first prescription, use of oral corticosteroids decreased by 9.1% (p=0.001), average daily dose decreased from 14.5 [18.4] mg to 11.9 [18.0] mg (p< 0.001) in the 6 months post initiation as compared to the 6 months prior. However, 48.6% of patients remained on a medium (7.5 mg – < 15 mg) or high dose (>15 mg). Initiation of belimumab resulted in no change in opioid use (Table 1).
Conclusion: These results suggest that a strikingly high proportion of patients with SLE are treated with corticosteroids to control the disease, and opioid therapy to manage chronic pain. While there was no change in opioid use, corticosteroid use decreased following initiation of belimumab.
Reference:
1. Chen SK et al. BMJ Open 2019;9:e027495
To cite this abstract in AMA style:
Birt J, Wu J, Griffing K, Bello N, Princic N, Winer I, Lew C, Costenbader K. Corticosteroid and Opioid Use Remain High in Systemic Lupus Erythematosus Patients Receiving Biologic Therapy: A Retrospective Claims Database Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/corticosteroid-and-opioid-use-remain-high-in-systemic-lupus-erythematosus-patients-receiving-biologic-therapy-a-retrospective-claims-database-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/corticosteroid-and-opioid-use-remain-high-in-systemic-lupus-erythematosus-patients-receiving-biologic-therapy-a-retrospective-claims-database-analysis/