Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The idiopathic inflammatory myopathies are a heterogeneous group of diseases that result in autoimmunity toward muscles and lead to tissue destruction, but the pathogenesis remains largely unknown. Synaptotagmin VII-knockout (Syt VII-/-) mice display mild myositis and we have previously demonstrated that combining this genetic defect with regulatory T-cell deficiency (FoxP3-/Y) results in a robust inflammatory myositis when adoptively transferred into immunodeficient (RAG1-/-) recipients. Interestingly, Syt VII-/- mice have impaired sarcolemmal membrane resealing capacity, which allows exposure of intracellular antigens. Tripartite motif (TRIM) proteins have also been linked to membrane repair capacity and are associated with myopathy in human patients. Here, we examined protein expression levels and subcellular localization of several novel TRIM proteins linked to membrane repair capacity in muscle tissue from mice using the Syt VII-/-/FoxP3-/Y model of myositis.
Methods: Membrane repair was monitored in vitro in cells using an established assay where the membrane of cultured cells is physically disrupted by glass microbeads. Mouse skeletal muscle was collected from wild type mice exercised on a treadmill or RAG1-/- mice adoptively transferred with lymph node preparations from Syt VII-/-/FoxP3-/Y mice. Tissue was analyzed by standard Western immunoblotting and by immunohistochemistry.
Results: We identified multiple TRIM family proteins that can modulate membrane repair capacity in cultured cells. Our results show that TRIM27 translocates to the membrane of injured muscle cells in vivo, as shown by immunohistochemistry. Similarly, when mice were exposed to membrane disruption due to eccentric contractions during treadmill running, there was translocation of TRIM27 from a diffuse pattern to the damaged membrane. In skeletal muscle of RAG1-/- mice, expression of several TRIM proteins, including TRIM27, was altered and displayed differential subcellular localization.
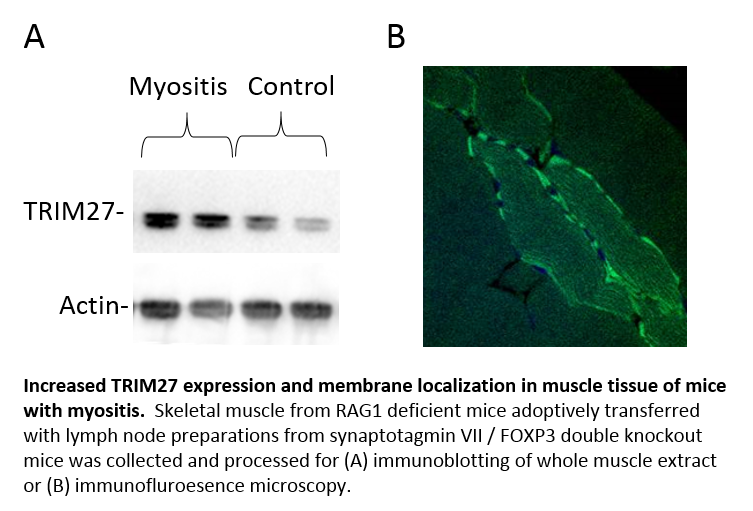 Conclusion: We have identified altered expression and localization of TRIM proteins in muscle in this mouse model of myositis. These results highlight an association of decreased sarcolemmal membrane integrity in the development of myositis and suggest a mechanism that could be targeted for diagnostics and therapeutics in these diseases.
Conclusion: We have identified altered expression and localization of TRIM proteins in muscle in this mouse model of myositis. These results highlight an association of decreased sarcolemmal membrane integrity in the development of myositis and suggest a mechanism that could be targeted for diagnostics and therapeutics in these diseases.
Disclosure:
J. Alloush,
None;
N. A. Young,
None;
K. McElhanon,
None;
W. N. Jarjour,
None;
N. Weisleder,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/contribution-of-tripartite-motif-proteins-modulating-membrane-repair-to-the-pathogenesis-of-autoimmune-mediated-myositis/
