Session Information
Date: Tuesday, November 14, 2023
Title: (2095–2140) RA – Diagnosis, Manifestations, and Outcomes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: RA-associated interstitial lung disease (ILD) and idiopathic pulmonary fibrosis (IPF) share genetic risk factors such as MUC5B rs35705950. The exact role of telomere related genes (TRG) in the RA-ILD genetic background is unclear. A previous work found an excess of TRG rare deleterious variants in RA-ILD compared to healthy controls but did not allow to conclude without a control population of patients with RA without ILD. Our aim was to test for association TRG rare deleterious exonic variants with ILD in patients with RA.
Methods: This genetic case-control association study was composed of a derivation (France) and a replication (US) population. Cases with RA-ILD and controls with RA without ILD (RA-noILD) that had whole exome sequencing (WES) data were included. ILD status and pattern (usual interstitial pattern, UIP or non-UIP) was determined by review of clinically-indicated high-resolution computed tomography (HRCT) chest imaging. Rare deleterious exonic variants from 14 candidate TRG previously associated with pulmonary fibrosis (TERT, TERC, PARN, RTEL1, CTC1, TINF2, ACD, POT1, NAF1, ZCCHC8, NHP2, NOP10, WRAP53, and DKC1) were selected using 1) a reported frequency in gnomAD database < 0.1%, 2) a deleterious impact predicted by SIFT, POLYPHEN-2, and 3) a CADD score > 15. Proportions of variants carriers in RA-ILD and RA-noILD were compared using a classical burden test adjusted for sex, age at RA, RA duration, MUC5B rs3570590 genotype and a principal component analysis using ancestry informative markers. Results were combined in a meta-analysis.
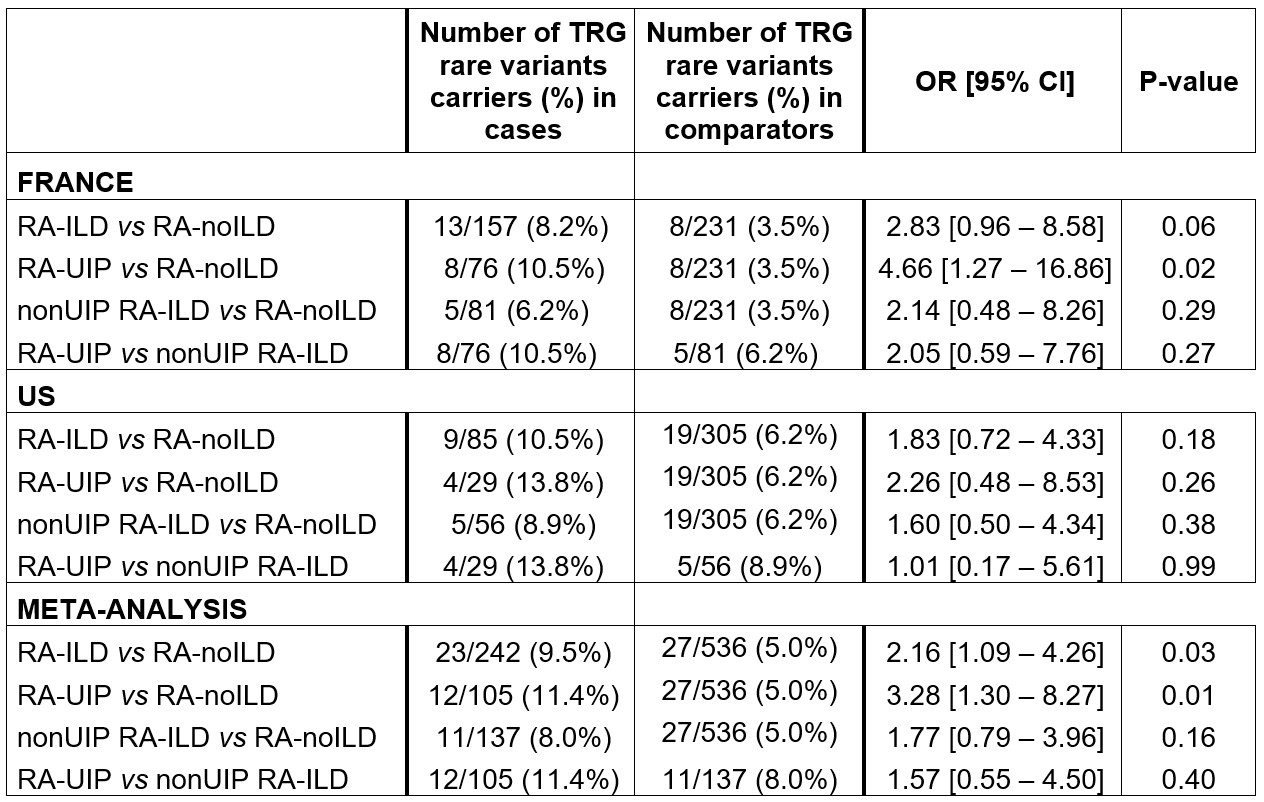
Results: The French derivation dataset included 388 patients, 157 cases (40.5%), mean age at ILD or last normal HRTC 60.1 years, 273 female (70.4%); Table 1. 22 rare deleterious variants were identified in 13/157 cases (8.3%) and 8/231 controls (3.5%). The burden test suggested an excess of TRG rare variants in RA-ILD (OR 2.83, 95% CI 0.96-8.58; p=0.06); (Table 2, Figure 1). A significant association was observed for usual interstitial pneumonia (UIP) HRCT pattern (OR 4.66, 95% CI 1.27-16.86; p=0.02) whereas no association was detected for patients with RA-ILD and a non-UIP HRCT patterns (OR 2.14, 95% CI 0.48-8.26; p=0.29). The US replication dataset included 390 patients, 85 cases (21.8%), mean age at ILD or last normal HRCT was 61.4 years and 74.4% were female. Similar trend for an excess of TRG rare variants was observed with 28 variants identified in 9/85 cases and 19/305 controls (10.5% vs 6.2%, OR 1.83, 95% CI 0.72-4.33; p=0.18); (Table 2, Figure 1). Like in the derivation population, the excess of rare variants was more pronounced for RA-UIP: 13.8% vs 6.2% (OR 2.26, 95% CI 0.48-8.53; p=0.26) The meta-analysis provide evidence for a significant contribution of TRGs rare variants to RA-ILD (OR 2.16, 95% CI 1.09-4.26; p=0.03), more specifically for the UIP pattern (OR 3.28, 95% CI 1.30-8.27; p=0.01). No significant association was found for non-UIP patterns in the meta-analysis (OR 1.77, 95% CI 0.79-3.96; p=0.16).
Conclusion: TRG rare deleterious exonic variants contribute to the risk of ILD in patients with RA, more specifically in RA-UIP. These results confirmed the shared genetic architecture between RA-ILD and IPF.
ACPA: anti-citrullinated peptides antibodies; HRCT: high resolution computed tomography; ILD: interstitial lung disease; MAF: minor allele frequency; RF: rheumatoid factor; UIP: usual interstitial pneumonia.
ILD: interstitial lung disease, OR [95% IC]: Odd ratios with 95% confidence interval; TRGs: telomere related genes; UIP: usual interstitial pneumonia.
Forest plot of odds ratios (OR) and 95% confidence intervals (CI). The boxes indicate OR, and the horizontal lines indicate 95% CI for the burden test comparing frequency of telomere related genes (TRG) rare deleterious exonic variants carriers in cases vs controls.
To cite this abstract in AMA style:
Juge P, Kawano-Dourado L, Gazal S, McDermott G, Hayashi K, Cui J, Debray M, Stervinou-Wemeau L, Marchand-Adam S, Richez C, Nunes H, Avouac J, Flipo R, Cottin V, Soubrier M, Saidenberg Kermanac'h N, Kannengiesser C, Borie R, Crestani B, Doyle T, Raychaudhuri S, Karlson E, Sparks J, Dieudé P. Contribution of Rare Deleterious Exonic Variants in Telomere Related Genes to Interstitial Lung Disease Risk in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/contribution-of-rare-deleterious-exonic-variants-in-telomere-related-genes-to-interstitial-lung-disease-risk-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/contribution-of-rare-deleterious-exonic-variants-in-telomere-related-genes-to-interstitial-lung-disease-risk-in-patients-with-rheumatoid-arthritis/