Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Baricitinib, an oral selective Janus kinase 1 and 2 inhibitor, has shown clinical efficacy and patient-reported pain relief in patients (pts) with RA and an inadequate response to conventional, synthetic DMARDs (RA-BEAM1) or biologic DMARDs (RA-BEACON2); and in DMARD-naïve pts (RA-BEGIN3). The objective of this post-hoc analysis is to quantify the contribution of pain relief to other patient-reported outcomes (PROs) using pooled data from pts who achieved control of inflammation in three phase 3 trials, regardless of treatment.
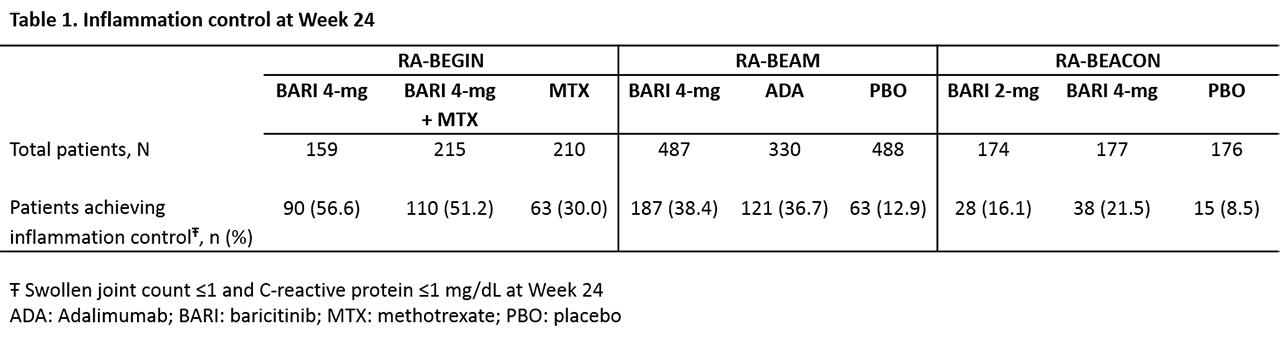
Methods: Well-controlled inflammation in RA was defined4 as swollen joint count (SJC) of 28 joints examined ≤1 and CRP ≤1 mg/dL at week 24. Among pts with well-controlled inflammation, PROs were compared between pts who also achieved thresholds of pain relief (defined as ≤20 mm or ≤40mm on a 0-100 mm visual analogue scale) at week 24 versus those who did not. PROs included: HAQ-Disability Index (HAQ-DI) normative value (< 0.5 for RA-BEAM and RA-BEACON, < 0.25 for RA-BEGIN) and minimum clinically important difference (MCID, ≥0.22), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) normative value (≥40.1) and MCID (≥3.56), and the 36-item Short Form Health Survey (SF-36) physical and mental component scores (PCS and MCS) MCID (≥5). Logistic regression models were adjusted for age, gender, body mass index, geographic region, duration of disease, and baseline SJC, CRP, pain, and value of the outcome. Missing data were imputed using modified last observation carried forward.
Results: Of the pts included in these phase 3 trials, 263 pts from RA-BEGIN, 371 pts from RA-BEAM, and 81 pts from RA-BEACON achieved inflammation control at week 24 (Table 1). Among pts who achieved inflammation control in RA-BEGIN and RA-BEAM, those who also achieved pain threshold ≤20 mm were more likely (p< 0.05) to report benefit in physical function (HAQ-DI), fatigue (FACIT-F), and general quality of life (SF-36) than pts who did not reach the pain relief threshold (Table 2). Generally, pts in RA-BEACON (inadequate responders to biologic DMARDs) were less likely to achieve all PRO thresholds than pts in other trials. In RA-BEACON, achieving pain ≤20 mm resulted in statistically greater percentages of pts achieving FACIT-F MCID, as well as HAQ-DI and FACIT-F normative values and numerically, but not statistically higher, percentages of pts for HAQ-DI MCID. The statistical insignificance may be due to the small number of RA-BEACON pts included in the analysis. Similar patterns were observed with the pain relief thresholds of ≤40 mm compared to >40 mm.
Conclusion: Despite apparently well-controlled inflammation (SJC ≤1 and CRP ≤1 mg/dL), residual pain may persist. Higher levels of pain >20mm are associated with worse physical function, fatigue, and quality of life. This may have implications for management decisions beyond treating to disease activity targets alone.
References: 1N Engl J Med. 2017, 376:652; 2N Engl J Med. 2016, 374:1243; 3Arthritis Rheumatol. 2017, 69:506; 4Arthritis Rheum. 2011, 63:573
To cite this abstract in AMA style:
van de Laar M, Pope J, Lee Y, Fautrel B, Ikeda K, Quebe A, Zhang X, Gaich C, De Leonardis F, Lisse J, Workman J, Fleischmann R, Genovese M, Taylor P. Contribution of Pain Relief to Function, Fatigue, and Quality of Life When Inflammation Is Controlled in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/contribution-of-pain-relief-to-function-fatigue-and-quality-of-life-when-inflammation-is-controlled-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/contribution-of-pain-relief-to-function-fatigue-and-quality-of-life-when-inflammation-is-controlled-in-patients-with-rheumatoid-arthritis/