Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Patients with juvenile idiopathic arthritis (JIA) have disease onset during childhood and often require anti-rheumatic medication through their reproductive years. Contraception use in reproductive-age females with inflammatory arthritis varies and has not been characterized in adults with JIA. We identified female adults with JIA in the American College of Rheumatology (ACR) Rheumatology Informatics System for Effectiveness (RISE) Registry and characterized their patterns of contraception.
Methods: We used data from the RISE Registry, a large, national electronic health record (EHR)-based registry to identify females age 18 to 45 years with > 2 ICD codes specific for JIA associated with clinical visits > 30 days apart during 2018-2019. Patients without any medication records were excluded. The primary outcome was documentation of contraception during the study period. Contraception information was extracted by national drug codes, current procedural terminology codes, international classification of disease codes, and medication names. Anti-rheumatic medications were categorized according to teratogenicity (Table 1). Patients’ sociodemographic information was also extracted. A generalized estimating equation (GEE) model estimated the association between anti-rheumatic medication teratogenicity and contraception use, as documented in the EHR, adjusting for age, race/ethnicity, geographic region, and Medicaid status.
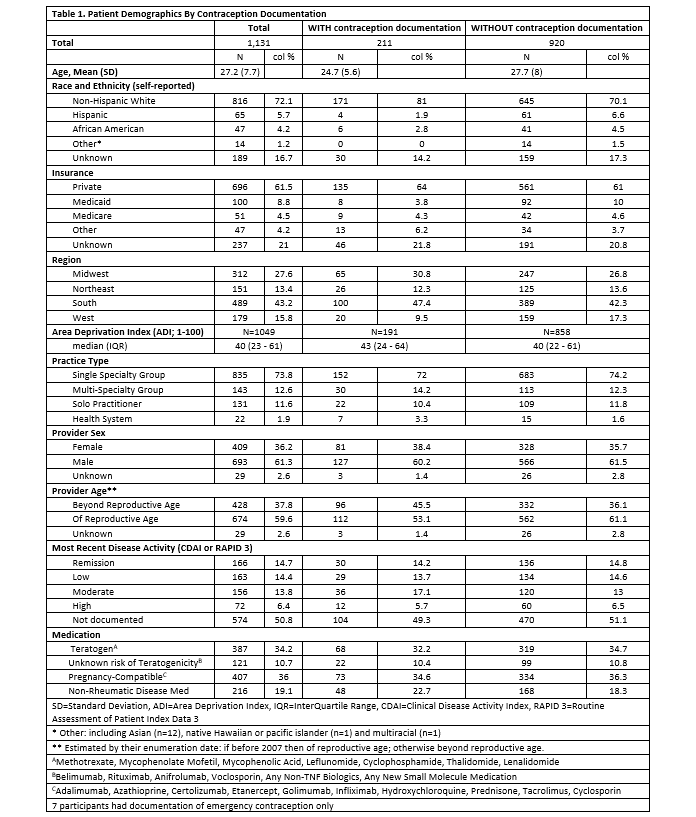
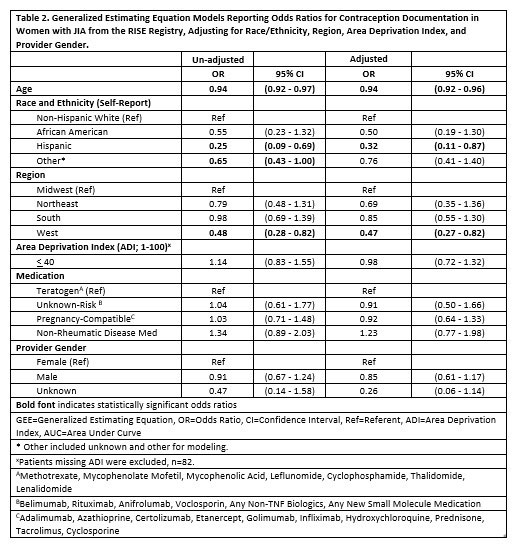
Results: 1,131 adult females were identified, with mean (SD) age 27.2 (7.7) and 72.1% non-Hispanic white (Table 1). 18.7% of participants had documentation of contraception. Similar documentation rates were observed by medication groups: 17.6% for teratogenic medication, 17.9% for pregnancy-compatible medication, 18.2% for unknown teratogenicity medication, and 22.2% for no anti-rheumatic medication. There was no significant relationship between medication teratogenicity and contraception documentation despite adjusting for other covariates (Table 2). Patients with Hispanic ethnicity, increased age, and those from the Western region were less likely to have contraception documented.
Conclusion: We found large gaps in contraception documentation within adult females with JIA within the RISE Registry. There was no identifiable relationship between contraception documentation and anti-rheumatic medication teratogenicity in JIA. Contraception documentation was significantly related to younger age, non-Hispanic ethnicity, non-Western geographic region, and non-Medicaid payer status. Future work should address how to increase contraception in this high-risk population and improve documentation of contraceptive medications and counseling.
Disclosures/Funding: These data were supported by the ACR’s RISE Registry and Rheumatology Research Foundation RISE Pilot Program award to Dr. John Bridges (952488). However, the views expressed represent those of the authors, not necessarily those of the ACR.
To cite this abstract in AMA style:
Bridges J, Li J, Mannion M, Schmajuk G, singh J. Contraception Documentation Patterns for Adult Females with Juvenile Idiopathic Arthritis on Teratogenic Medications in the Rheumatology Informatics System for Effectiveness (RISE) Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/contraception-documentation-patterns-for-adult-females-with-juvenile-idiopathic-arthritis-on-teratogenic-medications-in-the-rheumatology-informatics-system-for-effectiveness-rise-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/contraception-documentation-patterns-for-adult-females-with-juvenile-idiopathic-arthritis-on-teratogenic-medications-in-the-rheumatology-informatics-system-for-effectiveness-rise-registry/