Session Information
Date: Monday, November 13, 2023
Title: (1345–1364) Reproductive Issues in Rheumatic Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: It is important to control disease activity during pregnancy in women with rheumatic diseases because its exacerbation is associated with increased adverse pregnancy outcomes. Some of biologic disease-modifying anti-rheumatic drugs (bDMRARDs), especially in TNF inhibitors, are safe to continue during pregnancy, and become key drugs for controlling disease activity during pregnancy. However, there is little evidence whether continuing these drugs really does not affect unfavorable pregnancy outcomes, serious infections for mothers and infants, and adverse events of vaccination. Therefore, we investigated the effects of bDMARDs continuation on them.
Methods: We used the data of patients with rheumatic diseases who had been treated before pregnancy and gave birth in our institution. We extracted cases who were treated with bDMARDs at the time of conception, and divided them into two groups that discontinued bDMARDs at the time of pregnancy confirmed (discontinued group), and continued bDMARDs after pregnancy confirmed (continued group). We retrospectively examined pregnancy outcomes, serious infections requiring hospitalization for mothers and infants, live vaccination for infants in these two groups
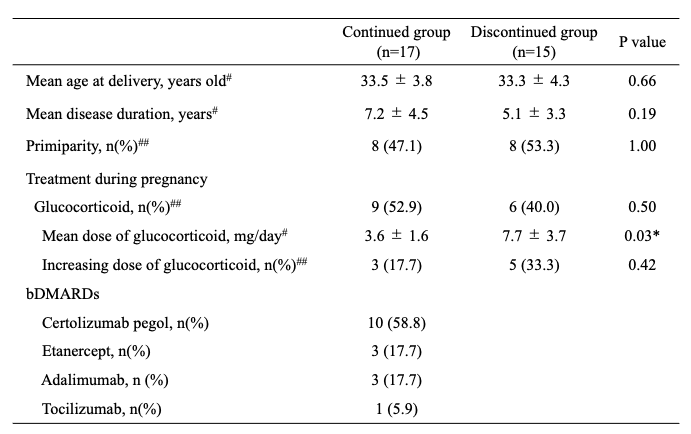
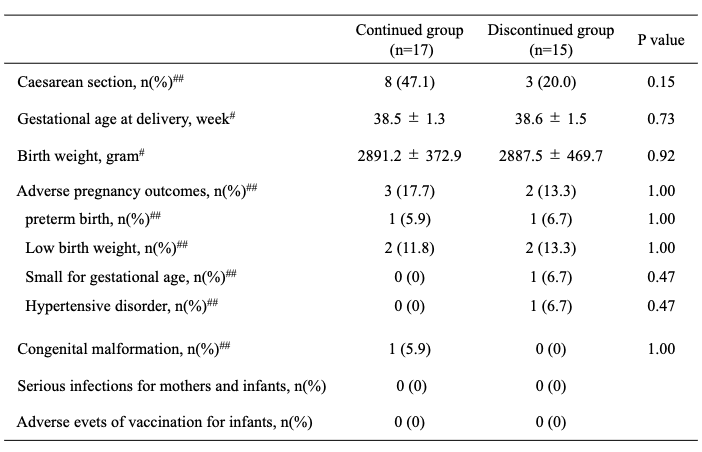
Results: Of 238 cases with rheumatic diseases who gave birth in our institution, 32 cases were treated with bDMARDs at the time of conception. Of these, 27 cases were rheumatoid arthritis, 3 cases were Behcet disease, 1 case was juvenile idiopathic arthritis, and 1 case was Takayasu aortitis, which were divided into 17 cases of the continued group, and 15 cases of discontinued group. Table 1 showed patients’ characteristics and treatment during pregnancy. In the continued group, certolizumab pegol was administered in 10 cases, etanercept was 3 cases, adalimumab was 3 cases, and tocilizumab was one case. All cases continued into second or third trimester with mean discontinued weeks of 28.5±5.1 weeks. In discontinued group, the mean dose of glucocorticoid was significantly higher compared to continued group (7.7±3.7 vs. 3.6±1.6, P=0.03). Table 2 showed pregnancy outcomes. The gestational weeks at delivery and birth weight in continued group were similar with discontinued group, and there was no significant difference of adverse pregnancy outcomes between these two groups. No serious infections requiring hospitalization were observed in either mother and infant in both groups. In addition, the infants received rotavirus vaccine from 2 months after birth, and BCG vaccine after 6 months according to the Japanese vaccine schedule, however, none of the infants had any adverse events due to vaccination.
Conclusion: In patients with rheumatic diseases, continuing bDMARDs had no association with the increase of unfavorable pregnancy outcomes, serious infections, and adverse events of vaccination. It was suggested that the continuation of bDMARDs may be an effective treatment option for rheumatic diseases during pregnancy. Larger number of examinations are needed as to how long to continue bDMARDs during pregnancy.
To cite this abstract in AMA style:
Shimada H, Wakiya R, Nakashima S, Miyagi T, Ushio Y, Sugihara K, Mino R, Mizusaki M, Chujo K, Kagawa R, Yamaguchi H, Kameda T, Dobashi H. Continuing Biologic DMARDs During Pregnancy in Women with Rheumatic Disease Was Not Associated with Increased Unfavorable Pregnancy Outcomes, Serious Infections, and Adverse Effects of Vaccination [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/continuing-biologic-dmards-during-pregnancy-in-women-with-rheumatic-disease-was-not-associated-with-increased-unfavorable-pregnancy-outcomes-serious-infections-and-adverse-effects-of-vaccination/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/continuing-biologic-dmards-during-pregnancy-in-women-with-rheumatic-disease-was-not-associated-with-increased-unfavorable-pregnancy-outcomes-serious-infections-and-adverse-effects-of-vaccination/