Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: In the INBUILD trial in patients with progressive fibrosing ILDs other than idiopathic pulmonary fibrosis (IPF), nintedanib reduced the rate of decline in forced vital capacity (FVC) over 52 weeks compared with placebo, with adverse events that were manageable for most patients. The safety and efficacy of nintedanib over longer-term use are being assessed in an open-label extension trial, INBUILD-ON. Here we present the safety and efficacy of nintedanib in patients with autoimmune disease-related ILDs in INBUILD-ON.
Methods: Patients in the INBUILD trial had diffuse fibrosing ILD (reticular abnormality with traction bronchiectasis, with or without honeycombing) of >10% extent on HRCT, FVC ≥45% predicted, DLco ≥30%–< 80% predicted, and met criteria for progression of ILD within the 24 months before screening, despite management deemed appropriate in clinical practice. Patients who completed the INBUILD trial on treatment (nintedanib or placebo) were eligible to enter INBUILD-ON. We analyzed adverse events and the change in FVC from baseline to week 60 of INBUILD-ON in the subgroup of patients with autoimmune disease-related ILDs based on a data snapshot taken on 15 October 2020. Analyses were descriptive.
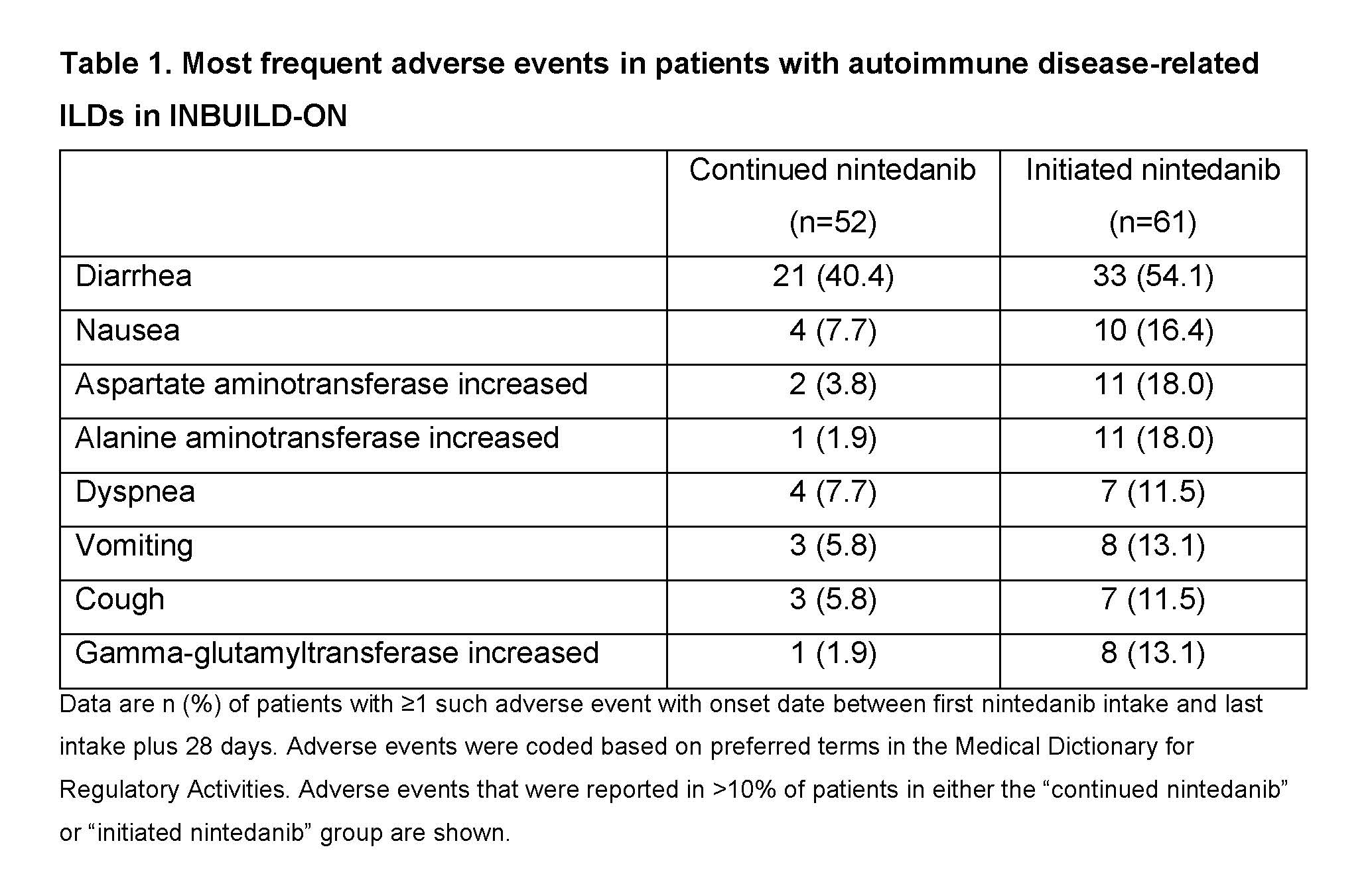
Results: Of the 434 patients treated in INBUILD-ON, 113 had autoimmune disease-related ILDs (52 RA-ILD, 29 SSc-ILD, 13 MCTD-ILD, 19 other autoimmune disease-related ILDs). Of these, 61.9% took ≥1 disease-modifying anti-rheumatic drug or high-dose glucocorticoid during the trial. Diarrhea was the most frequent adverse event (Table 1). Adverse events led to discontinuation of nintedanib in 11.5% of patients who continued nintedanib in INBUILD-ON (having taken nintedanib in INBUILD) and 29.5% of patients who initiated nintedanib in INBUILD-ON (having taken placebo in INBUILD). The adverse event that most frequently led to discontinuation of nintedanib was diarrhea (Table 2). Mean (SE) changes in FVC from baseline to week 60 of INBUILD-ON were −77.6 (41.1) mL in patients who continued nintedanib (n=32), −32.8 (29.3) mL in patients who initiated nintedanib (n=32) and −55.2 (25.2) mL in all patients (n=64), similar to the change from baseline to week 52 of the INBUILD trial in patients with autoimmune disease-related ILDs who received nintedanib (−78.8 [29.5] mL).
Conclusion: The adverse event profile of nintedanib in patients with autoimmune disease-related ILDs participating in INBUILD-ON was characterized mainly by gastrointestinal events and was consistent with that reported over 52 weeks in INBUILD. These data support the manageable safety profile of nintedanib over continued use in patients with autoimmune disease-related ILDs.
To cite this abstract in AMA style:
Matteson E, Antin-Ozerkis D, Bonella F, Chaudhuri N, Cottin V, Mueller H, Coeck C, Rohr K, Wuyts W. Continued Treatment with Nintedanib in Patients with Progressive Fibrosing Autoimmune Disease-Related Interstitial Lung Diseases: Data from INBUILD-ON [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/continued-treatment-with-nintedanib-in-patients-with-progressive-fibrosing-autoimmune-disease-related-interstitial-lung-diseases-data-from-inbuild-on/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/continued-treatment-with-nintedanib-in-patients-with-progressive-fibrosing-autoimmune-disease-related-interstitial-lung-diseases-data-from-inbuild-on/