Session Information
Date: Tuesday, November 9, 2021
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II (1862–1888)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Painful, recurring oral ulcers (OU) associated with Behçet’s syndrome negatively affect quality of life (QoL). Differences across sexes were reported in the frequency of disease manifestations, disease course, and response to colchicine. The phase 3, randomized, double-blind, placebo (PBO)-controlled RELIEF study showed overall efficacy of apremilast (APR) for OU associated with Behçet’s syndrome, including improvements in OU pain, disease activity, and QoL. The objective of this subgroup analysis is to evaluate the consistency of efficacy with APR in men and women with Behçet’s syndrome.
Methods: Adults with active Behçet’s syndrome and ≥3 OU at randomization or ≥2 OU at screening and randomization, without active major organ involvement, were randomized to APR 30 mg BID or PBO during the 12-week PBO-controlled treatment phase. Randomization was stratified by sex. The primary endpoint was area under the curve for the number of OU through Week 12 (AUCWk0-12) to assess continued efficacy over the time period in a symptom that waxed and waned. Key secondary endpoints included OU pain, complete response (OU-free), maintenance of complete response, and QoL at Week 12. Disease activity was also assessed using Behçet’s Syndrome Activity Score (BSAS) and Behçet’s Disease Current Activity Index Form (BDCAF). QoL was assessed using Behçet’s Disease QoL (BDQoL). Prespecified subgroup analyses in men and women were performed to assess treatment effect in primary and secondary endpoints.
Results: Eighty men and 127 women were randomized and received ≥1 dose of study medication. Mean age was 38.7 years (men) and 40.8 years (women). Mean (SD) OU count at baseline was 3.4 (1.4) (PBO) and 3.7 (1.5) (APR) for men and 4.3 (3.2) (PBO) and 4.5 (4.5) (APR) for women. Greater improvements in favor of APR vs PBO were observed in AUCWk0-12 in men and women (Figure). Consistency in efficacy with APR was observed between men and women, with greater reduction in pain and achievement of OU complete response (OU-free) and maintenance of response at Week 12 vs PBO (Table). In men and women, consistent treatment effects in favor of APR vs PBO were observed for disease activity and QoL measures, although moderate treatment differences were observed in BDCAI (men/women) and BDQoL (men) (Table).
Conclusion: Consistent treatment effects in favor of APR vs PBO in clinically relevant outcomes, including OU number and pain, OU complete response, and disease activity measures, were observed in men and women with OU associated with Behçet’s syndrome.
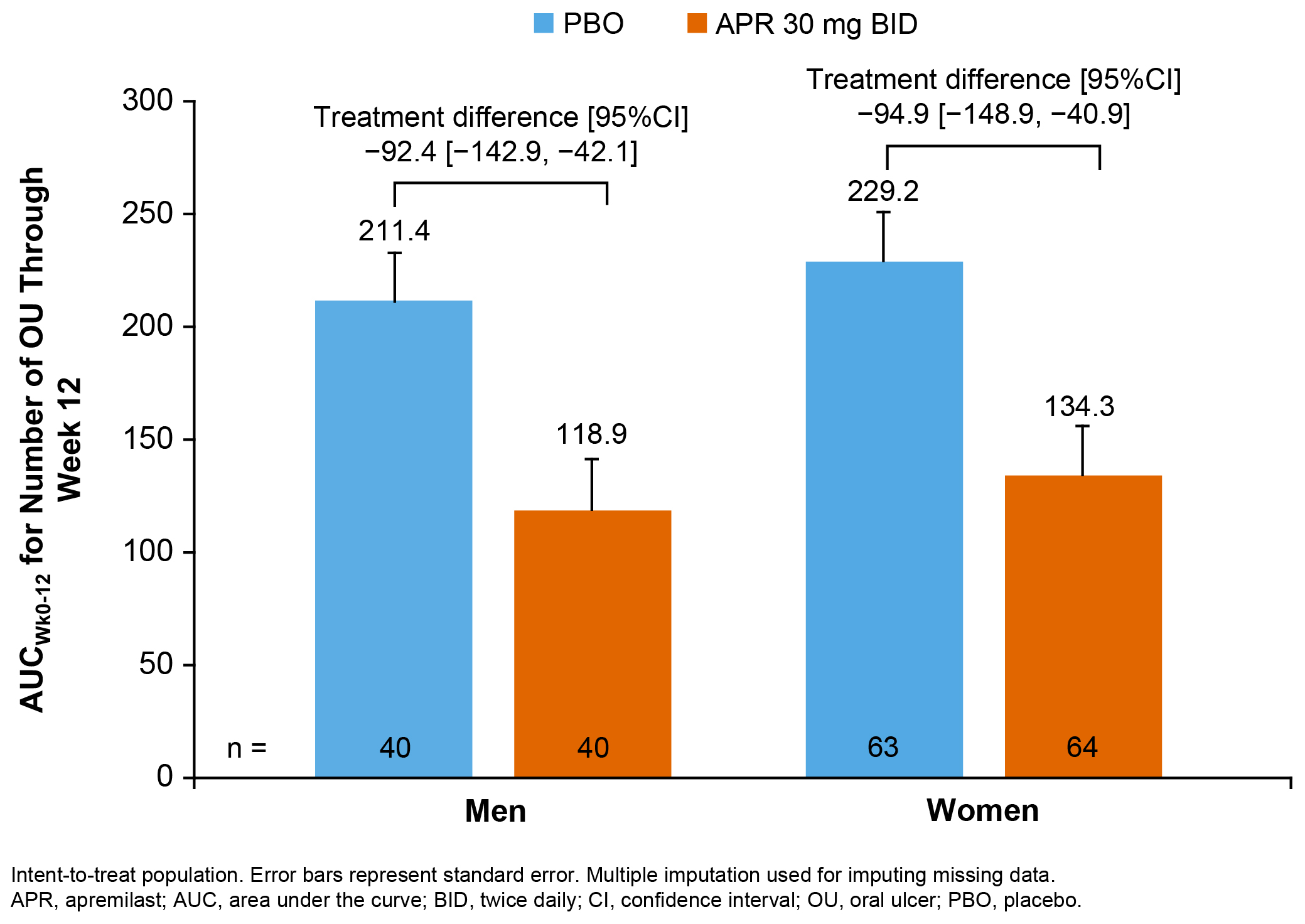 Area Under the Curve for the Number of OU From Baseline Through Week 12 (Primary Endpoint)
Area Under the Curve for the Number of OU From Baseline Through Week 12 (Primary Endpoint)
To cite this abstract in AMA style:
Hatemi G, Mahr A, Takeno M, Kim D, Melikoğlu M, Cheng S, Richter S, Jardon S, Paris M, Chen M, Yazici Y. Consistent Efficacy with Apremilast in Men and Women to Treat Oral Ulcers Associated with Behçet’s Syndrome: Results from Phase 3 Researching Oral Apremilast Safety and Efficacy in Behçet’s Disease (RELIEF) Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/consistent-efficacy-with-apremilast-in-men-and-women-to-treat-oral-ulcers-associated-with-behcets-syndrome-results-from-phase-3-researching-oral-apremilast-safety-and-efficacy-in-behcets-d/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consistent-efficacy-with-apremilast-in-men-and-women-to-treat-oral-ulcers-associated-with-behcets-syndrome-results-from-phase-3-researching-oral-apremilast-safety-and-efficacy-in-behcets-d/

