Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Upadacitinib (UPA) has demonstrated efficacy in patients with moderate-to-severe rheumatoid arthritis (RA) across various patient populations.1–4 This post hoc analysis aimed to evaluate the consistency in time to achieving meaningful clinical response with UPA 15 mg + conventional synthetic (cs) DMARDs in biologic (b) DMARD-inadequate responder (IR) versus csDMARD-IR patients with RA as well as with UPA 15 mg monotherapy versus UPA 15 mg + csDMARDs in csDMARD-IR patients.
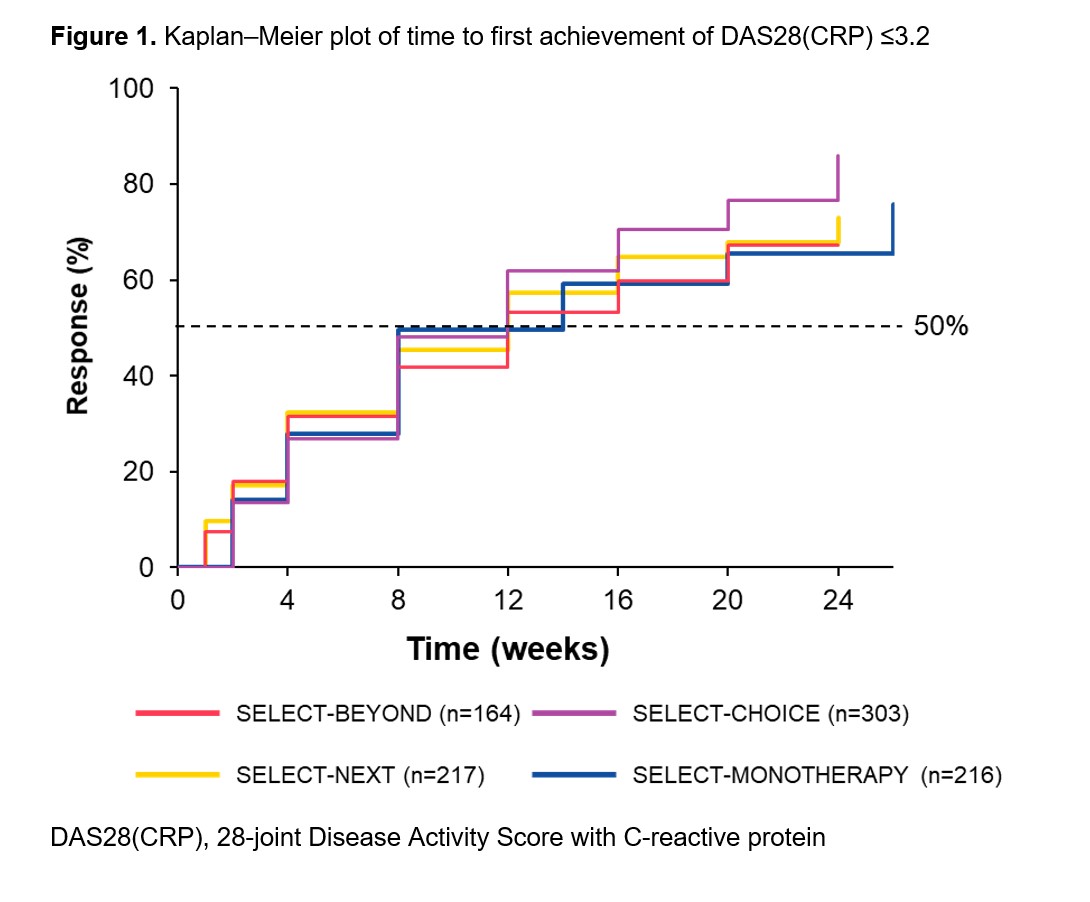
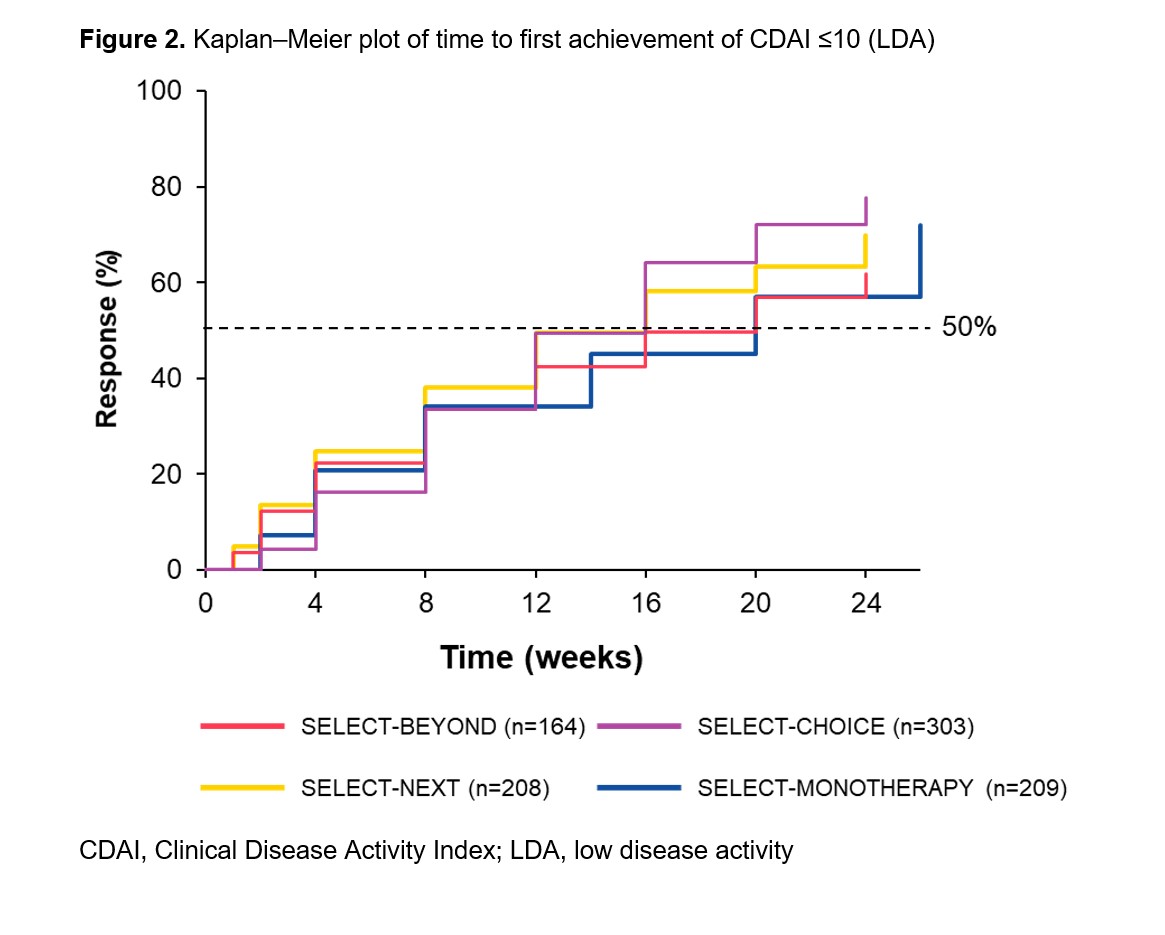
Methods: Patients originally randomized to UPA 15 mg once daily from four Phase 3 trials were included in this analysis: SELECT-BEYOND1 and SELECT-CHOICE2 (UPA 15 mg + csDMARDs in bDMARD-IR patients), SELECT-NEXT3 (UPA 15 mg + csDMARDs in csDMARD-IR patients), and SELECT-MONOTHERAPY4 (UPA 15 mg monotherapy in methotrexate-IR patients). Time to response was estimated using the Kaplan–Meier method for clinical outcomes over 24 weeks (26 weeks in SELECT-MONOTHERAPY). Clinical outcomes included achievement of 28-joint Disease Activity Score with C-reactive protein (DAS28[CRP]) ≤3.2; low disease activity (LDA) defined as Clinical Disease Activity Index (CDAI) ≤10 and Simple Disease Activity Index (SDAI) ≤11; and 50% improvement in American College of Rheumatology (ACR) core components and morning stiffness (MS) duration/severity. Data presented were as observed.
Results: Overall, 905 patients were included (SELECT-BEYOND: n=164; SELECT-CHOICE: n=303; SELECT-NEXT: n=221; SELECT-MONOTHERAPY: n=217). csDMARD-IR patients had a mean disease duration of 7.3 (SELECT-NEXT) or 7.5 years (SELECT-MONOTHERAPY); bDMARD-IR patients had a mean disease duration of 12.4 years, with a more refractory population (≥3 prior bDMARDs) in SELECT-BEYOND (23%) than SELECT-CHOICE (10%). In general, the median time to DAS28(CRP) ≤3.2, CDAI LDA, 50% improvement in ACR core components, and 50% improvement in MS duration/severity were consistent across the studies in bDMARD-IR and csDMARD-IR patients. For SELECT-BEYOND, SELECT-CHOICE, SELECT-NEXT, and SELECT-MONOTHERAPY, the median (95% CI) time to achieve DAS28(CRP) ≤3.2 was 12 (12, 16), 12 (8, 12), 12 (8, 12), and 14 (8, 14) weeks, respectively (Figure 1), and the median time to achieve CDAI LDA was 20 (12, 24), 16 (12, 16), 16 (12, 16), and 20 (14, 20) weeks, respectively (Figure 2). A longer median (95% CI) time to achieve SDAI LDA was observed with UPA monotherapy (20 [14, 20] weeks) versus UPA + csDMARDs (12 [12, 16] weeks) in csDMARD-IR patients. Among bDMARD-IR patients, the median (95% CI) time to 50% improvement in pain was longer in SELECT-BEYOND versus SELECT-CHOICE (16 [12, 20] versus 8 [8, 12] weeks).
Conclusion: In diverse patient populations with RA, patients treated with UPA 15 mg, as monotherapy or with csDMARDs, generally demonstrated consistent time to achieving DAS28(CRP) ≤3.2, CDAI LDA, and 50% improvement in clinical outcomes.
- Genovese MC, et al. Lancet 2018;391:2513–24.
- Rubbert-Roth A, et al. N Engl J Med 2020;383:1511–21.
- Burmester GR, et al. Lancet 2018;391:2503–12.
- Smolen JS, et al. Lancet 2019;393:2303–11.
To cite this abstract in AMA style:
Rubbert-Roth A, Combe B, Szekanecz Z, Hall S, Haraoui B, Attar S, Ekwall A, Song Y, Shaw T, Nagy O, Xavier R. Consistency in Time to Response with Upadacitinib as Monotherapy or Combination Therapy and Across Patient Populations with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/consistency-in-time-to-response-with-upadacitinib-as-monotherapy-or-combination-therapy-and-across-patient-populations-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consistency-in-time-to-response-with-upadacitinib-as-monotherapy-or-combination-therapy-and-across-patient-populations-with-rheumatoid-arthritis/