Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: In the last decade, treatment with tumor necrosis factor (TNF) inhibitors has significantly improved the outcome in patients with rheumatoid arthritis (RA). Recent studies have highlighted drug immunogenicity as a mechanism behind treatment failure. Concomitant immunosupressors, such as methotrexate (MTX), can reduce the production of anti-drug anitibodies (ADA) which might reduce drug efficacy and result in drug discontinuation. We investigated the effect of concomitant use of MTX on the long-term adherence of etanercept and adalimumab.
Methods: All eligible patients were registered in the Tsurumai Biologics Communication Registry (TBCR), an RA research consortium that consists of Nagoya University Hospital and 12 affiliated institutes. As of September 2011, 2,176 RA patients treated with biologics are registered in this registry. Seven hundred ninety four RA patients previously unexposed to biological DMARDs were treated with etanercept (n = 560) or adalimumab (n = 234). Drug discontinuation rates were calculated by Kaplan-Meier method using the end-point of inefficacy or adverse events. Statistical difference between two groups was tested by Log-rank test. Statistical significance was defined as p-value < 0.05.
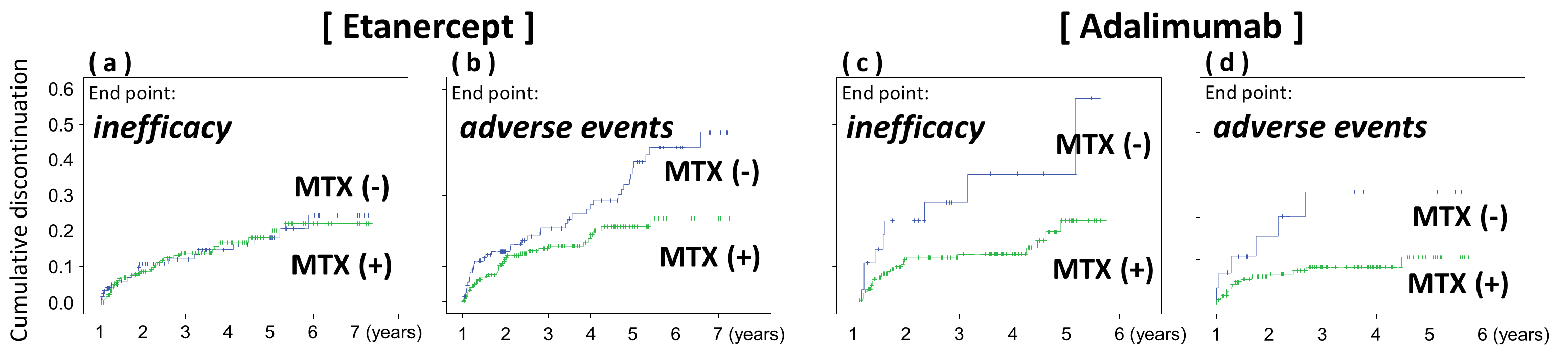
Results: Among the etanercept group, the discontinuation rate due to inefficacy were quite similar between the patients with concomitant MTX (n = 385) and those without (n = 175) (16.8 vs 14.8% at 3 years and 22.2 vs 24.5% at 6 years, p = 0.936, Fig. a). Conversely among the adalimumab group, the patients with concomitant MTX demonstrated significantly lower discontinuation rate due to inefficacy (13.5 vs 36.2% at 3 years, p = 0.014, Fig. c). The patients without MTX demonstrated significantly higher discontinuation rate due to adverse events both in the etanercept (p = 0.011, Fig. b) and adalimumab group (p = 0.016, Fig. d). The reason for this difference would have included the patients’ background complications for which they could not take MTX therapy. Unfortunately, such information was not available in the registry data.
Conclusion: It was quite interesting that the concomitant MTX usage did not improve the discontinuation rate due to inefficacy in the patients with etanercept therapy. Production of anti-drug antibodies (ADA) reduces the biological DMARDs effect that can be attenuated by concomitant methotrexate, which reduces ADA frequency. In the case of adalimumab, some previous reports demonstrated that the long-term clinical outcome is strongly dependent on the presence or absence of anti-adalimumab antibodies. On the other hand, some report showed that anti-etanercept antibody is hardly detected. Our current data clearly support these previous data that showed less immunogenicity of etanercept. Thus, we would conclude that the drug immunogenicity should be considered especially for the patients that cannot take concomitant MTX.
Disclosure:
N. Takahashi,
None;
T. Kojima,
None;
A. Kaneko,
None;
Y. Hirano,
None;
N. Ishiguro,
Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, BMS, Eisai, Janssen, Kaken and Pfizer,
2,
Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, BMS, Eisai, Janssen, Kaken, Pfizer, Taisho-Toyama and Otsuka,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/concomitant-methotrexate-did-not-affect-discontinuation-rate-of-etanercept-due-to-ineffectiveness-six-year-results-from-japanese-multicenter-registry-system/