Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Recent studies implicate complement activation in the pathophysiology of antiphospholipid syndrome (APS), especially in patients with severe manifestations, such as catastrophic APS (CAPS). This study aimed to prospectively evaluate whether complement activation biomarkers are associated with new (first or recurrent) thrombosis in antiphospholipid antibody (aPL)-positive patients.
Methods: APS ACTION registry inclusion criteria are positive aPL based on the updated Sapporo APS Classification Criteria, tested at least twice within one year prior to enrollment. Patients are prospectively followed every 12±3m with clinical data and blood collection. We identified patients with new thrombosis during follow-up, and controls without new thrombosis, matched (1:1) for gender, age (±5 years), history of thrombosis, and associated autoimmune disease. Complement activation was evaluated by plasma levels of soluble C5b-9 (sC5b-9), C4d, and Bb fragment (commercial ELISA), and the modified HAM (mHAM) assay that measures complement-dependent cell killing. For each patient, two annual follow-up samples were evaluated (first available and most recent prior to event; or first two available in those without new thrombosis). If available, a third “acute” sample within three months of the new thrombosis was also studied.
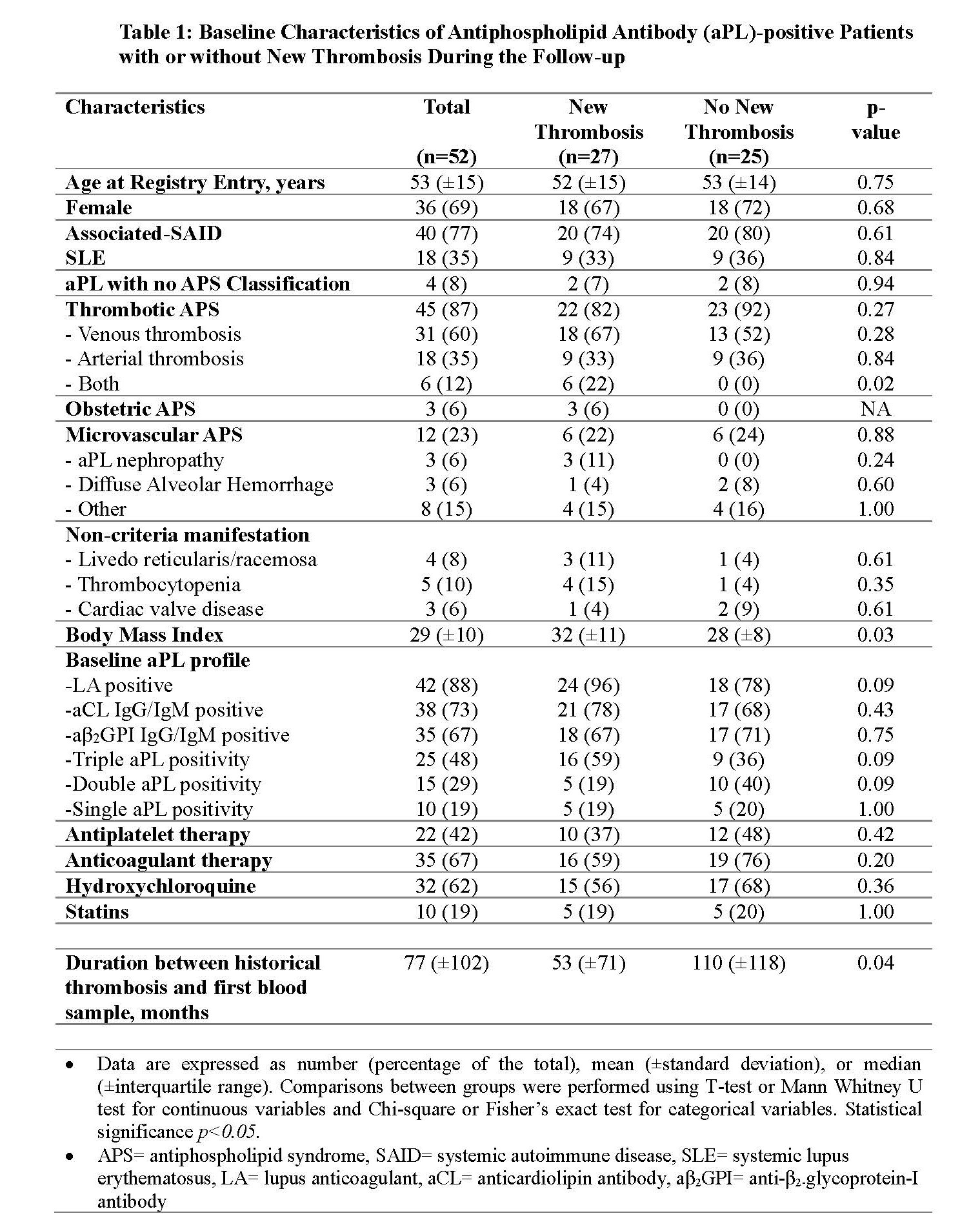
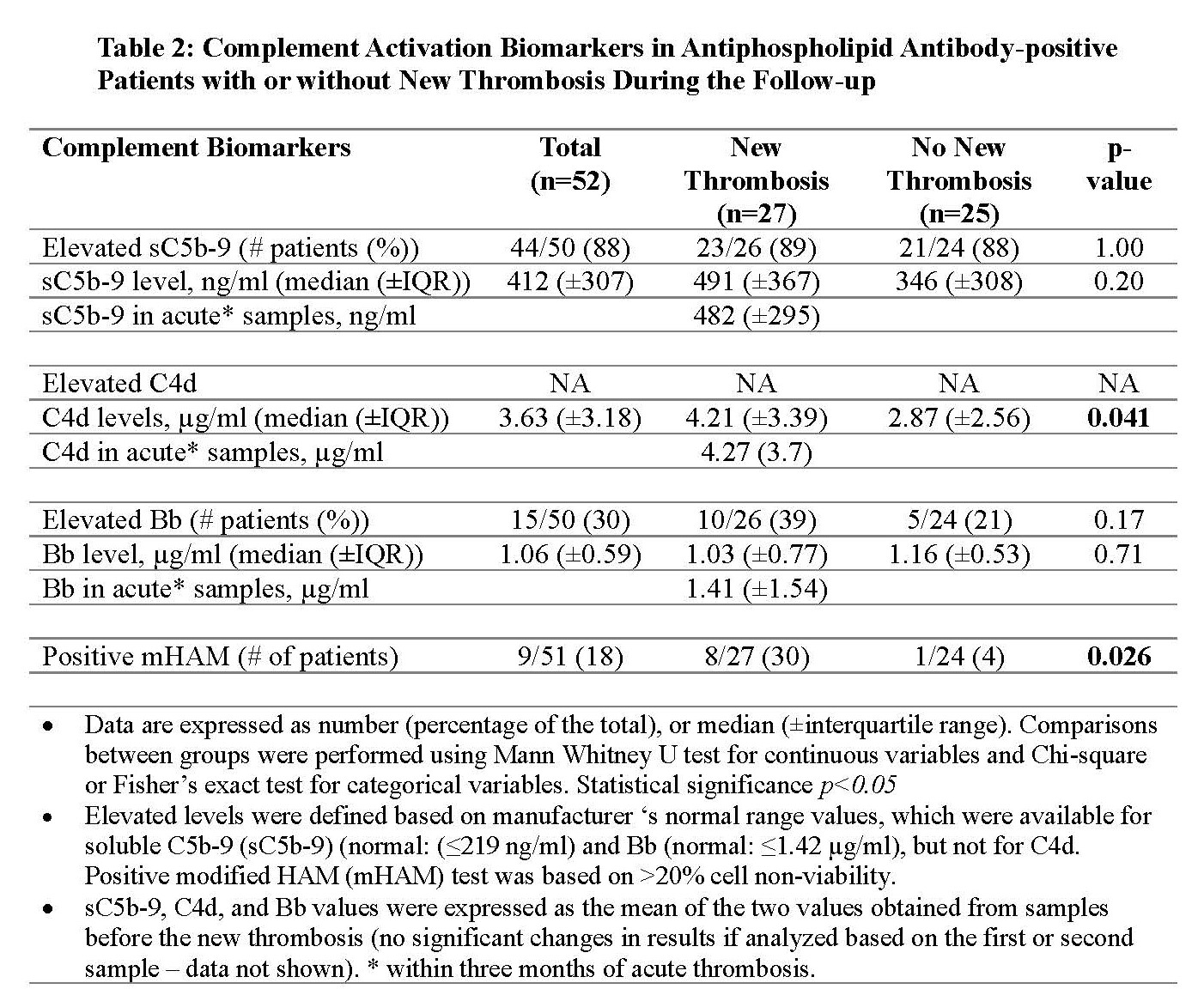
Results: As of May 2022, 365 aPL-positive patients from North American centers were included in the registry; 27 (7%) patients had a new thrombosis during the prospective follow-up and were matched with 25 patients without new thrombosis. Baseline characteristics were similar, except those with new thrombosis were more likely to have a history of both arterial and venous thromboses (p=0.02), a higher body mass index (p=0.03), and a trend towards triple aPL positivity (p=0.09) (Table 1). After a median follow-up of 4.6 years (±IQR, 5.6), 13 patients had new arterial thrombosis, 12 venous, and two microvascular. In patients with new thrombosis, compared to those with no new thrombosis: a) C4d level was significantly elevated (median level 4.22 (±IQR, 3.39) µg/ml versus 3.33 (±IQR, 2.56) µg/ml; p=0.041); b) the number of patients with positive mHAM test was significantly higher (8 [30%)]versus 1 [4%]; p=0.026); and c) there was no significant difference in sC5b-9 and Bb levels despite a trend toward a higher number of elevated Bb in patients with new thrombosis (Table 2). In nine patients with “acute” samples obtained within three months of new thrombosis, sC5b-9 was elevated in all, Bb fragment in four (44%), and mHAM test was positive in three (33%).
Conclusion: Based on the analysis of our multi-center prospective cohort of persistently aPL-positive patients, our preliminary findings suggest that markers of complement activation, specifically elevated C4d levels and positive mHAM test, are associated with a higher risk of thrombosis, which might be a useful tool to aid risk-stratification.
To cite this abstract in AMA style:
Yelnik C, Chaturvedi S, Labreuche J, Pan X, Belmont H, Nina K, Fortin P, Branch D, Zuo Y, Willis R, Brodsky R, Salmon J, Bertolaccini M, Cohen H, Petri M, Erkan D, Of APS ACTION O. Complement Activation as a Marker of Thrombosis Risk in Antiphospholipid Antibody Positive Patients: Prospective Results from AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/complement-activation-as-a-marker-of-thrombosis-risk-in-antiphospholipid-antibody-positive-patients-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-n/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/complement-activation-as-a-marker-of-thrombosis-risk-in-antiphospholipid-antibody-positive-patients-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-n/