Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Recent RA-interstitial lung disease (RA-ILD) treatment guidelines noted a paucity of evidence on the comparative effectiveness and safety of DMARDs in RA-ILD. Previously, TNFi were not associated with worse outcomes than non-TNFi b/tsDMARDs in RA-ILD, though subgroup analyses suggested findings may differ for specific b/tsDMARDs. We compared outcomes between different non-TNFi b/tsDMARDs in RA-ILD using the Target Trial Emulation Framework.
Methods: We emulated three two-arm trials by comparing abatacept, tocilizumab, and tofacitinib with rituximab, a common treatment used for connective tissue disease (CTD)-ILD. Patients with RA-ILD were identified in national Veterans Affairs (VA) data (2006-2020) with validated algorithms, and b/tsDMARD courses were assembled from pharmacy data. Prior receipt of ILD therapies (e.g., cyclophosphamide, mycophenolate, antifibrotics) and diagnoses for other CTDs or lymphoproliferative disease were exclusions. Pairwise propensity score matching (1:1) was used to separately balance abatacept, tocilizumab, and tofacitinib initiators to rituximab (reference). Models included demographics, comorbidities and general health indicators, RA-related measures (e.g., prior DMARDs, elevated acute phase reactants, autoantibody status), and ILD-related measures (including forced vital capacity [FVC]). Cox regression models adjusting for unbalanced variables (SMD >0.1) were used to analyze the primary composite outcome of death (VA, National Death Index) and respiratory-related hospitalization (VA, Medicare) over up to a 3-year follow-up period via an intention-to-treat approach. Secondary analyses assessed the individual components of the composite outcome. Sensitivity analyses were performed with alternative analytic approaches and among cohorts with modified eligibility criteria.
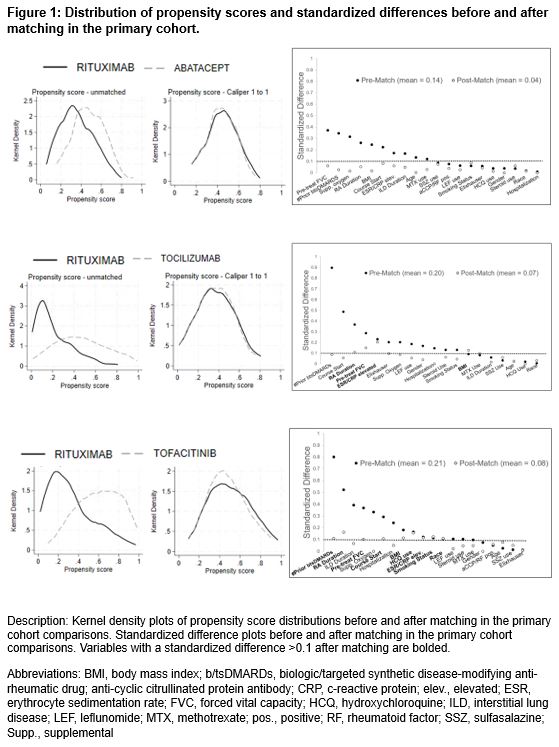
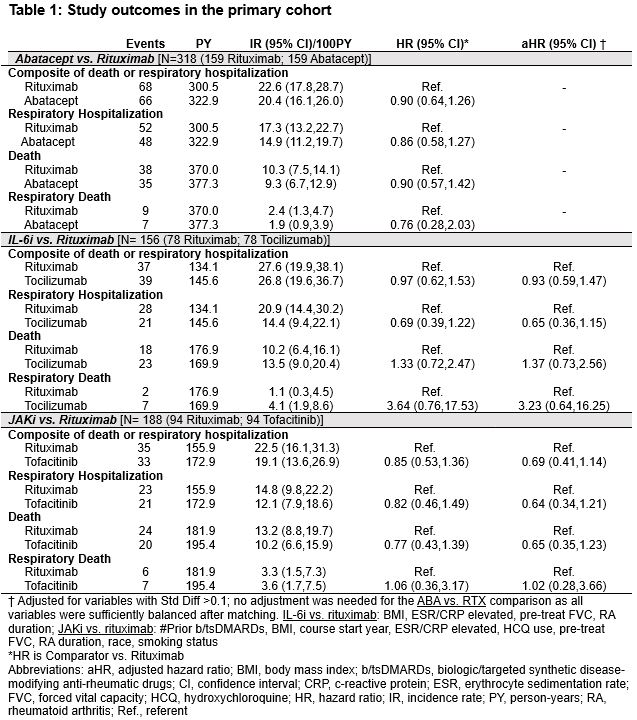
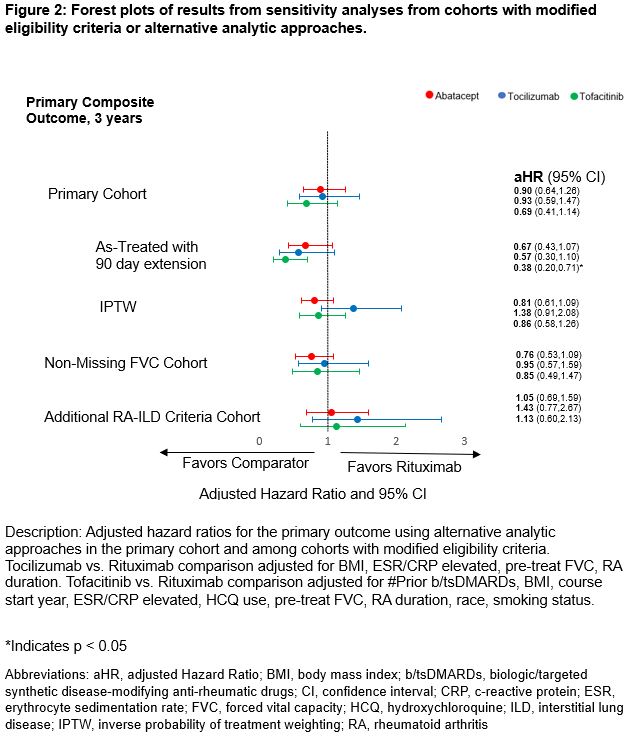
Results: In the primary cohort, we matched abatacept (n=159), IL-6i (n=78), and JAKi (n=94) with equal numbers of rituximab initiators. Cohorts had a mean age of 68.1-69.9 years, were mostly male (range 87-91%), and baseline FVC was 74-80% predicted. All variables were balanced in comparisons of abatacept vs. rituximab, while some remained imbalanced in other comparisons (Figure 1). There were no significant differences in the primary composite outcome among any of the comparisons (vs. rituximab: abatacept HR 0.90 [0.64, 1.26]; tocilizumab aHR 0.93 [0.59, 1.47]; tofacitinib aHR 0.69 [0.41, 1.14]) (Table 1, Figure 2). Similarly, there were no significant differences in the individual outcomes among these comparisons (Table 1). Sensitivity analyses in cohorts with modified eligibility criteria and using inverse probability of treatment weighting supported these findings with the exception of as-treated analyses that favored the comparator over rituximab (Figure 2).
Conclusion: We did not observe significant differences in outcomes over a 3-year follow-up between RA-ILD patients initiating abatacept, tocilizumab, or tofacitinib versus rituximab, though estimates were imprecise. These real-world findings emphasize the need for clinical trials of advanced immunomodulatory therapies in RA-ILD.
To cite this abstract in AMA style:
Frideres H, Wichman C, Dong J, Roul P, Yang Y, Baker J, George M, Johnson T, Rojas J, brian S, Cannon g, Matson S, Curtis J, Mikuls T, England B. Comparisons of Non-TNFi Biologic and Targeted Synthetic DMARDs in Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Propensity Score-Matched Study Using National Veterans Affairs Data [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparisons-of-non-tnfi-biologic-and-targeted-synthetic-dmards-in-rheumatoid-arthritis-associated-interstitial-lung-disease-a-propensity-score-matched-study-using-national-veterans-affairs-data/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparisons-of-non-tnfi-biologic-and-targeted-synthetic-dmards-in-rheumatoid-arthritis-associated-interstitial-lung-disease-a-propensity-score-matched-study-using-national-veterans-affairs-data/