Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: To compare the efficacy and tolerance at 3 and 6 months of two methotrexate (MTX) initiation strategies in rheumatoid arthritis (RA).
Methods: Retrospective,monocentric,cross-sectionalstudy including patients with RA who initiated MTX as first-line therapy during the last 2 years according to one of the following 2 strategies: a “conventional” strategy (CS) defined by an initiation of oral MTX at a dose of 10-15 mg/week or an “aggressive” strategy (AS), defined by an initiation of subcutaneous (SC) MTX at a dose of 15 mg/week SC or >15 mg/week either orally or SC. Each strategy allowed the possibility to increase the doses and/or switch to the SC route at 3 months. Efficacy was assessed at 3 and 6 months using the DAS28-CRP. The tolerance of each strategy was also assessed at month 3 and 6.
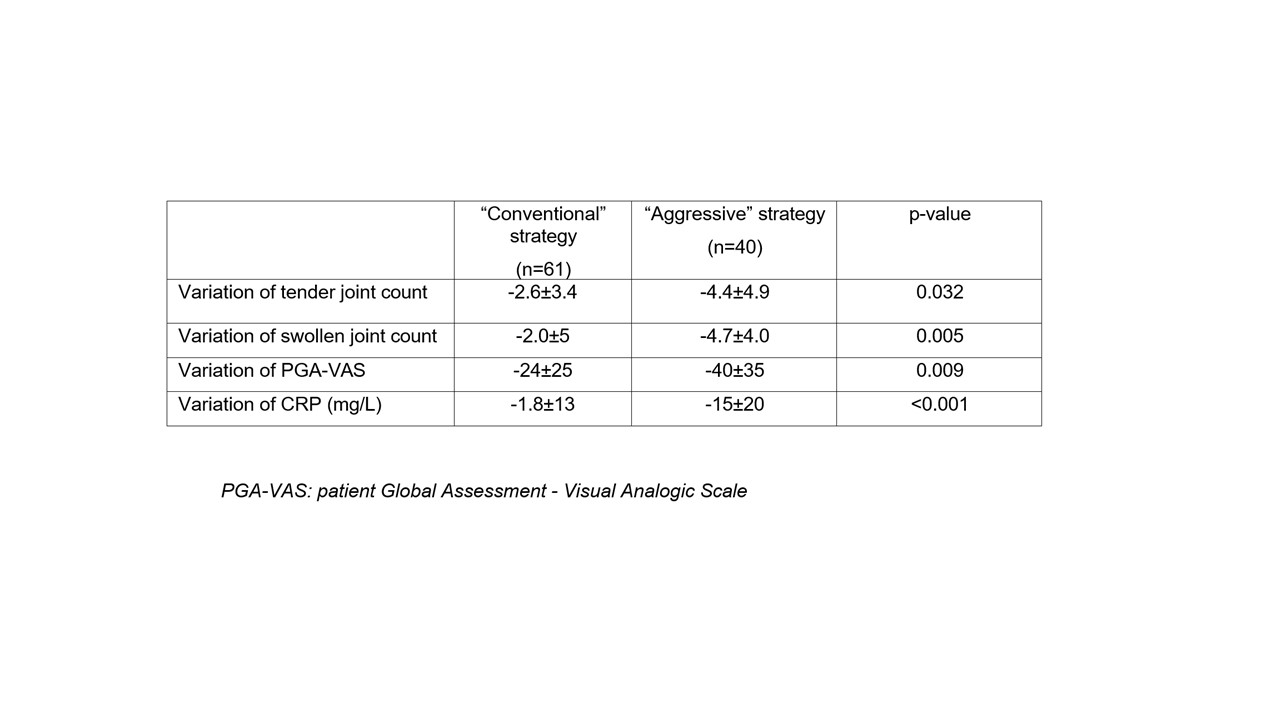
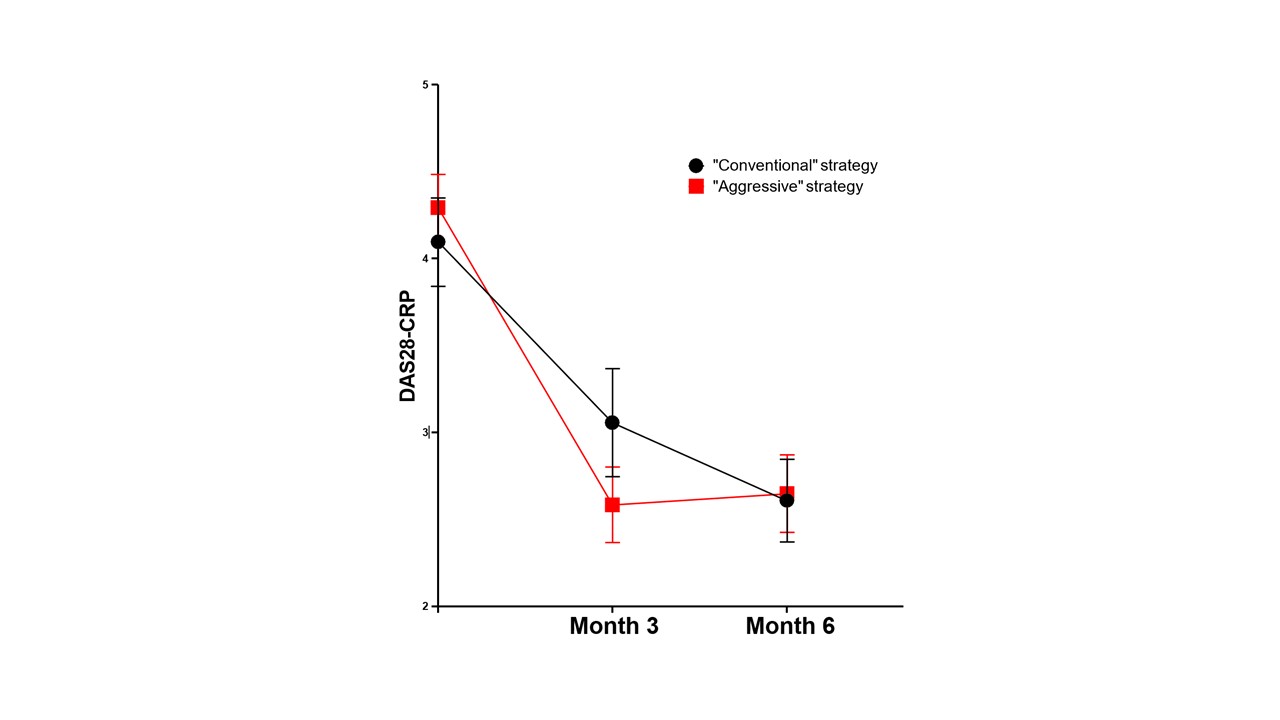
Results: We included 101 patients (85 women) with a mean age of 55±12 years and disease duration of 5±6 months. The frequency of rheumatoid factors, anti-CCP antibodies and erosions was 83%, 81% and 38% respectively. 61 patients initiated MTX according to the CS, with an increase of dose and/or a switch to the SC route at 3 months for 31 patients, and 40 patients started treatment according to the AS, with an increase of dose and/or switch to the SC route at 3 months for 14 patients. There was no difference between these 2 groups in terms of age, gender, disease duration, antibody status, frequency of bone erosions, body mass index, comorbidities and disease activity at baseline. Efficacy at 3 months was significantly higher with the AS (reduction of the DAS28-CRP from 4.34±0.91 to 2.39±0.75, mean difference of 1.95±1.21, p< 0.001) compared to the CS (reduction of the DAS28-CRP from 4.09±0.62 to 2.88±0.73, mean difference of 1.21±0.90, p=0.12) (Figure 1). The improvement of tender/swollen joint counts, patient global assessment and CRP levels was also significantly more important at 3 months with the AS (Table 1). At 6 months, although the DAS28-CRP was similar in the 2 groups (Figure 1), less patients from the AS subgroup required an escalation to a targeted biologic/synthetic therapy compared to the CS (12/40, 30% vs. 29/61, 48%, p=0.073). The frequency of digestive side effects at 3 months was significantly lower in the AS (3/40, 7,5% vs. 16/61, 26%, p=0.021). The frequency of hepatic cytolysis at 3 month was higher in the AS (4/40, 10% vs. 1/61,1,6%, p=0.057). The frequency of asthenia at 3 months was similar in both groups (7/4, 18% vs. 6/61, 10%, p=0.25). Only one infection was observed in the CS and no hematological side effect was recorded. At 6 months, the cumulative incidence of side effects was 23% with the AS compared to 46% with the CS (p=0.015). Only one treatment discontinuation was noted in the AS subgroup vs. 9 in the CS subgroup (p=0.042).
Conclusion: This study suggests that it is possible to use a more aggressive initiation strategy of MTX in RA in routine clinical practice. This strategy allows to obtain an earlier clinical response and it is associated with a better tolerance than the conventional strategy. These results need to be confirmed in prospective studies.
Figure 1: Evolution at 3 and 6 months of DAS28-CRP index according to the methotrexate initiation strategy
To cite this abstract in AMA style:
Vidal-Montal P, Combier A, STEELANDT A, THOMAS M, Narvaez J, Nolla J, ALLANORE Y, AVOUAC J. Comparison of Two Methotrexate Initiation Strategies in Rheumatoid Arthritis in Current Practice [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparison-of-two-methotrexate-initiation-strategies-in-rheumatoid-arthritis-in-current-practice/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-two-methotrexate-initiation-strategies-in-rheumatoid-arthritis-in-current-practice/