Session Information
Date: Tuesday, November 15, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Measurement of plasma anti-TNF concentration, in combination with the assessment of clinical outcomes, may be used to optimize dosing regimens for individual patients (pts). For successful implementation in clinical practice, an assessment of the different quantitative methods available is necessary. Here, we compare two enzyme-linked immunosorbent assays (ELISAs) for the quantitative measurement of plasma certolizumab pegol concentration ([CZP]), using data from the RAPID-PsA trial of CZP in pts with psoriatic arthritis (PsA).
Methods: Pts in RAPID-PsA (NCT01087788) were treated with a CZP loading dose (LD; 400 mg at Weeks [Wks] 0, 2, 4), followed by a maintenance dose (200 mg Q2W or 400 mg Q4W). Plasma samples were taken at baseline, Wks 2, 4, 12, 16, 24, 48, 72 and 96. A bespoke ELISA developed by UCB Pharma, validated in line with FDA/EMA regulatory requirements for bioanalytical methods (assay range: 0.4–33.3 μg/mL), was used to measure plasma [CZP] between 2010–2013. In 2016, a subset of frozen plasma samples was reanalyzed with the LISA-TRACKER® validated diagnostic kit (current range: 0.4–12.0 μg/mL). Paired CZP measurements were plotted and the degree of agreement between both assays was evaluated by the Bland-Altman method. Reproducibility of plasma [CZP] measurement with LISA-TRACKER® was evaluated by measuring in duplicate a set of 207 plasma samples from a different CZP study (NCT01500278).
Results: 917 paired CZP measurements from RAPID-PsA pts were analyzed. Plasma [CZP] measured with LISA-TRACKER® showed good correlation with measurements obtained with the UCB Pharma ELISA, particularly for [CZP] between 10–100 μg/mL; [CZP] was highest at Wk 4, corresponding to the end of the LD period (Figure A). Bland-Altman analysis revealed a mean ratio LISA-TRACKER®/UCB Pharma ELISA of 1.19 (95% CI 1.13–1.25); the majority of paired measurements differed less than 3-fold from each other (Figure B). [CZP] <10 μg/mL were associated with greater variability. Reproducibility analysis showed a coefficient of variation of 13.5% for LISA-TRACKER® [CZP] measurements. Observed differences between assays should be interpreted with caution, as LISA-TRACKER® measurements were performed ≥3 years after the original analysis; over time, suboptimal sample storage conditions may have increased the difference in [CZP] detected by the two methods.
Conclusion: CZP concentrations measured with LISA-TRACKER®, a commercially available diagnostic kit, were 19% higher than those measured with the bespoke ELISA developed by UCB Pharma. The two assays showed good agreement, suggesting that data measured with both assays can be extrapolated to clinical practice. 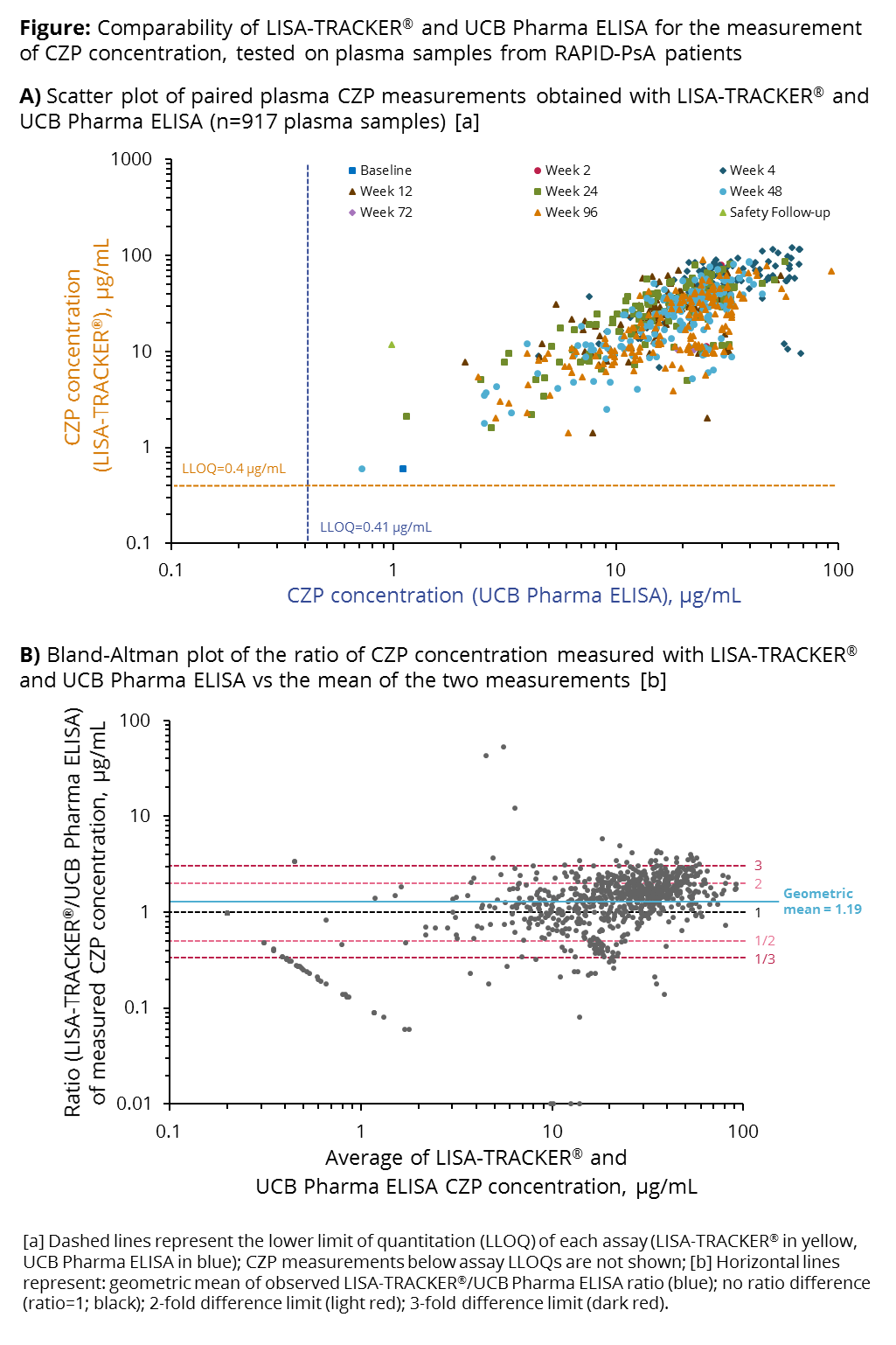
To cite this abstract in AMA style:
Paul S, Smeraglia J, de Longueville M, O'Brien C, Parussini E. Comparison of Two Enzyme-Linked Immunosorbent Assays Used for Drug Concentration Monitoring in Psoriatic Arthritis Patients Treated with Certolizumab Pegol [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/comparison-of-two-enzyme-linked-immunosorbent-assays-used-for-drug-concentration-monitoring-in-psoriatic-arthritis-patients-treated-with-certolizumab-pegol/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-two-enzyme-linked-immunosorbent-assays-used-for-drug-concentration-monitoring-in-psoriatic-arthritis-patients-treated-with-certolizumab-pegol/
