Session Information
Date: Sunday, November 5, 2017
Title: Rheumatoid Arthritis – Clinical Aspects Poster I: Treatment Patterns and Response
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
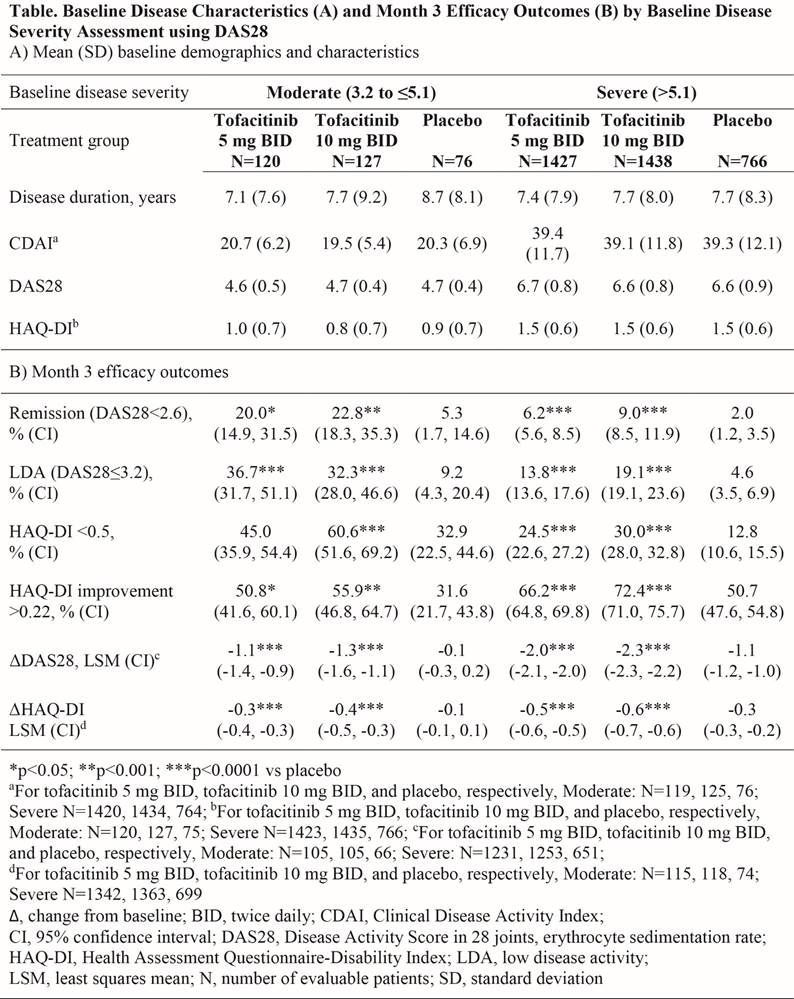
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). We evaluated tofacitinib 5 and 10 mg twice daily (BID) efficacy in patients (pts) with moderate vs severe RA.
Methods: Tofacitinib 5 and 10 mg BID efficacy data were from 6 randomized, double-blind Phase 3 studies of 6–24 months’ (Mos) duration. Pts received tofacitinib as monotherapy (NCT00814307 ORAL Solo; NCT01039688 ORAL Start) or with csDMARDs, mainly MTX (NCT00960440 ORAL Step; NCT00847613 ORAL Scan; NCT00856544 ORAL Sync; NCT00853385 ORAL Standard). Pts receiving MTX monotherapy (ORAL Start) or placebo (PBO) (±csDMARDs) were combined as a single PBO group. Baseline (BL) disease severity was classified as moderate or severe using Disease Activity Score in 28 joints, erythrocyte sedimentation rate (DAS28: moderate 3.2–≤5.1; severe >5.1) and Clinical Disease Activity Index (CDAI; moderate 10–≤22; severe >22). Mo 3 efficacy outcomes included: pts (%) achieving low disease activity (LDA; DAS28≤3.2, CDAI ≤10), remission (REM; DAS28<2.6, CDAI ≤2.8), HAQ-DI <0.5 (normal physical functioning), HAQ-DI improvement >0.22, and mean change from BL (Δ) in DAS28, CDAI, and HAQ-DI. This post-hoc analysis had no multiplicity adjustments.
Results: More pts had severe disease at BL by DAS28 (91.8%) and CDAI (90.7%). Mo 3 efficacy outcomes for pts were classified by BL DAS28 disease severity (Table). BL characteristics were balanced between treatment groups in each disease severity category (Table). In general, Mo 3 efficacy was significantly greater for tofacitinib 5 and 10 mg BID vs PBO, regardless of BL disease severity. Larger proportions of tofacitinib-treated pts with moderate vs severe BL RA achieved LDA by either DAS28 (32.3–36.7% vs 13.8–19.1%) or CDAI (49.2–55.0% vs 26.0–31.7%). A higher proportion of pts achieved REM in the moderate vs severe BL groups by DAS28 (20.0–22.8% vs 6.2–9.0%) or CDAI (11.5–12.1% vs 5.1–6.7%). A greater proportion of pts achieved HAQ‑DI <0.5 with moderate vs severe RA classified by BL DAS28 (45.0–60.6% vs 24.5–30.0%) or BL CDAI (40.8–52.4% vs 24.7–30.4%). Pts with severe vs moderate RA had greater improvements from BL in disease activity and HAQ-DI when classified by BL DAS28 (Table), and by BL CDAI (tofacitinib 5/10 mg BID ΔCDAI: -21.1/-23.0 vs -8.1/-9.4; ΔHAQ‑DI: -0.5/-0.6 vs -0.3/-0.4).
Conclusion: Tofacitinib 5 and 10 mg BID demonstrated efficacy in treating pts with moderate and severe RA with >7 years’ mean disease duration. By Mo 3, pts with severe vs moderate BL disease activity had greater improvements in disease activity and physical functioning; higher proportions of pts with moderate vs severe BL disease activity achieved REM, LDA, or normal physical functioning. This post-hoc analysis may be limited by the smaller sample size of the moderate disease group and the combining of mono- and combination-therapy results.
To cite this abstract in AMA style:
Schwartzman S, Sunkureddi P, Takiya L, Snyder M, Fan H, Lukic T, Roberts J, Rigby WFC. Comparison of Tofacitinib Efficacy in Patients with Moderate Vs Severe Rheumatoid Arthritis: Pooled Analysis of Phase 3 Studies [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparison-of-tofacitinib-efficacy-in-patients-with-moderate-vs-severe-rheumatoid-arthritis-pooled-analysis-of-phase-3-studies/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-tofacitinib-efficacy-in-patients-with-moderate-vs-severe-rheumatoid-arthritis-pooled-analysis-of-phase-3-studies/