Session Information
Date: Monday, November 6, 2017
Title: Health Services Research Poster II: Osteoarthritis and Rheumatoid Arthritis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Previous HA studies have focused on the Medicare population, but less is known of the treatment patterns and cost of HA relative to knee arthroplasty (KA) and other therapies for younger privately insured patients. We evaluated the overall cost of treating knee OA in a large group of Blue Cross/Blue Shield (BCBS) patients, including those from KA and non-arthroplasty therapies, such as HA, corticosteroid injections, and physical therapy. We hypothesized that non-arthroplasty interventions would account for the majority of knee OA costs in the younger patient population.
Methods: Knee OA-related claims were identified from Blue Health Intelligence claims data (2011-2015). The dataset contains claims for 140+ million unique BCBS members nationwide in the U.S. Cumulative costs (adjusted to November 2016$) were evaluated from a payor perspective and grouped into various categories, such as physical therapy, corticosteroid injections, HA, and KA.
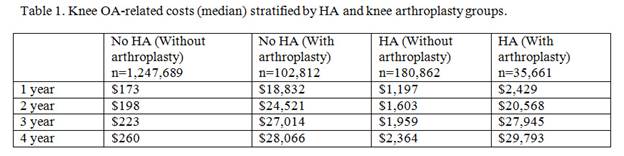
Results: The overall cost of treating knee OA for 1,567,024 knee OA patients over the five-year period was $5.7B (average $3,600/patient). HA accounted for $196.9M (3.5%) of the overall cost, based on 216,523 HA patients (13.8% of knee OA cohort). KA accounted for $3.6B (63.5%) of the overall costs, while office visits, arthroscopy, anesthesia for knee surgery, arthrocentesis, knee imaging, and physical therapy accounted for $229.3M (4.0%), $141.8M (2.5%), $140.8M (2.5%), $124.6M (2.2%), $115.0M (2.0%), and $51.9M (0.91%), respectively. 16.5% of the HA patients subsequently underwent knee arthroplasty during the study period, but HA contributed to 2.8% of their overall knee OA treatment costs compared to KA, which contributed 82.9%. For those who received KA, the median costs for the HA cohort were lower at 1 and 2 years compared to the no-HA cohort, but similar at 3 years and marginally higher at 4 years (Table 1). If the 180,862 HA patients who avoided KA during the study period had, instead, undergone arthroplasty, the cost of KA would be estimated to total $4.8B. Instead, there was a cost savings of $4.3B (90.1%) by utilizing non-arthroplasty therapies.
Conclusion: Non-arthroplasty therapies accounted for about a third of the costs (36.5%) in treating knee OA in our cohort of younger patients. Interventions that were not recommended or determined to have inconclusive evidence by AAOS accounted for 3.5% (HA), 0.12% (corticosteroid), and 0.54% (knee brace) of the overall costs. Although questions have been raised about the effectiveness of HA, the majority of HA patients avoided knee arthroplasty during the study period; they saved an estimated 90.1% by utilizing non-arthroplasty therapies. With the wide spectrum of therapies to treat knee OA, efforts to identify the most appropriate candidates for arthroplasty and non-arthroplasty therapies, such as HA or other non-HA therapies, can help reduce costs to the healthcare system.
To cite this abstract in AMA style:
Ong K, Niazi F, Lau E, Shaw P, Kurtz S. Comparison of the Costs for Hyaluronic Acid and Total Knee Arthroplasty in the Treatment of OA for the Blue Cross/Blue Shield Patient Population [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparison-of-the-costs-for-hyaluronic-acid-and-total-knee-arthroplasty-in-the-treatment-of-oa-for-the-blue-crossblue-shield-patient-population/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-the-costs-for-hyaluronic-acid-and-total-knee-arthroplasty-in-the-treatment-of-oa-for-the-blue-crossblue-shield-patient-population/