Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The EULAR and ACR clinical guidelines recommend switching to a different disease-modifying antirheumatic drug (DMARD) when biologic-treated patients experience treatment failure or toxicity. Lack of efficacy and adverse events are among the most commonly reported reasons for switching biologic therapies. Limited information is available regarding biologic therapy persistence across subcutaneously administered (SC) biologic agents in the real-world setting, as well as comparative information on biologic persistence for SC biologics among patients with rheumatoid arthritis (RA) who are not naïve to biologic treatment. This study compared the persistence of SC biologic DMARDs (bDMARDs) in patients with RA as subsequent-line therapy following a failure of first-line bDMARDs.
Methods: US administrative claims data were used to create a longitudinal cohort of adult patients with RA initiating SC biologic between 1/1/2012 and 6/30/2017 (initiation date = index). Patients were required to have failed 1st bDMARD to enter study (but could later switch therapy) and to have 6 and at least 3 months of continuous enrollment pre- and post-index (date of prescription for bDMARD). Those with other autoimmune conditions were excluded from the study. Outcomes were biologic persistence, defined as number of days between initiation date and last supplied day of last fill. Parametric survival models with exponential distribution with robust variance estimator were used to compare outcomes for tocilizumab versus other biologics, adjusting for differences in baseline characteristics, accounting for correlation among different bDMARD episodes.
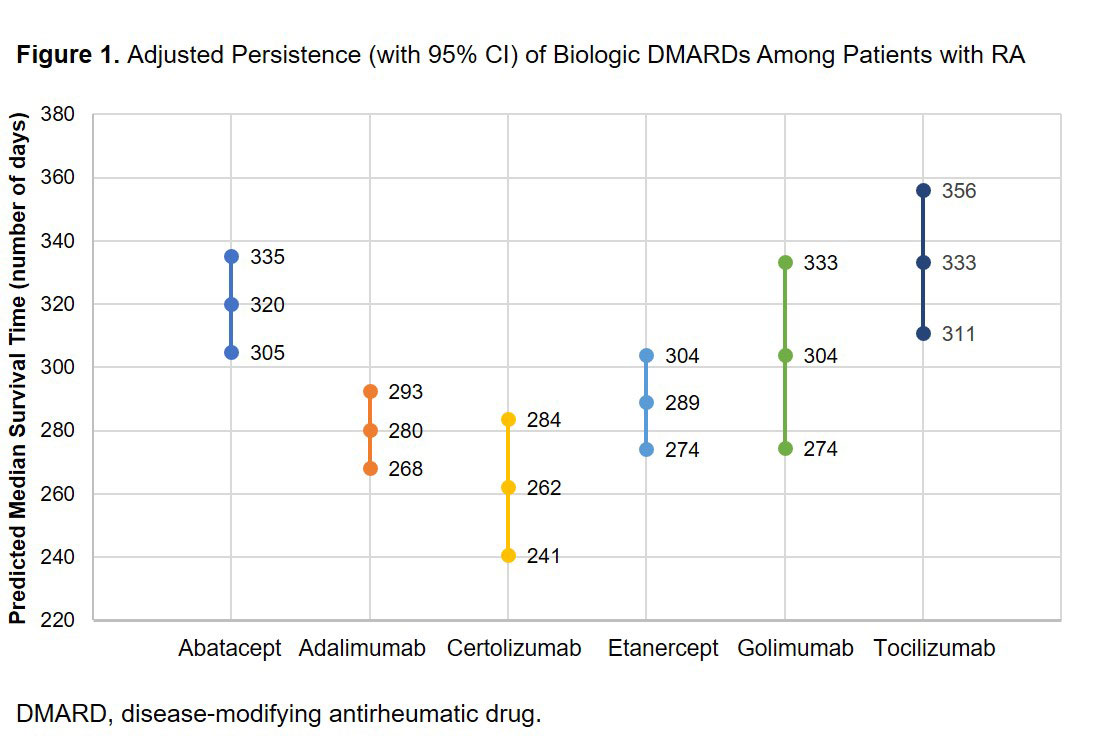
Results: There were 10,301 patients with 12,704 bDMARD episodes: abatacept (n = 2,988), adalimumab (n = 3,599), certolizumab (n = 982), etanercept (n = 2,760), golimumab (n = 745), or tocilizumab (n = 1,630). Mean age ranged from 51.0 to 53.3 years. Mean [SD] Elixhauser comorbidity scores were significantly higher (P < 0.001) for tocilizumab (2.8 [2.3]) compared with abatacept (2.5 [2.2]), adalimumab (2.5 [2.1]), certolizumab (2.4 [2.0]), etanercept (2.4 [2.0]) or golimumab (2.4 [2.2]). Adjusted median days (95% CI) of persistence were: abatacept 320 (305, 335); adalimumab 280 (268, 293); certolizumab 262 (241, 284); etanercept 289 (274, 304); golimumab 304 (274, 333); and tocilizumab 333 (311, 356). Tocilizumab had significantly (P < 0.05) higher persistence compared with adalimumab, certolizumab and etanercept (Figure 1). Of patients who were observed for 12 months, 45% of patients initiated tocilizumab biweekly and 55% initiated weekly. Of the 347 patients initiating biweekly tocilizumab, 33% switched to weekly over 12-month follow up; the mean time to switch was 177 days. After 12 months of follow-up, approximately 68% of patients finished on weekly dosing and 32% on biweekly dosing.
Conclusion: Among patients with RA who previously used ≥ 1 other biologic, tocilizumab-treated patients had similar or statistically significantly better biologic persistence compared with other biologics.
Acknowledgements: This study was funded by Genentech, Inc.
To cite this abstract in AMA style:
Best J, Tominna L, Abbass I. Comparison of Real-World Persistence of Subcutaneously Administered Biologic Disease-Modifying Antirheumatic Drug Therapies Among Patients with Rheumatoid Arthritis Switching from Another Biologic [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparison-of-real-world-persistence-of-subcutaneously-administered-biologic-disease-modifying-antirheumatic-drug-therapies-among-patients-with-rheumatoid-arthritis-switching-from-another-biologic/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-real-world-persistence-of-subcutaneously-administered-biologic-disease-modifying-antirheumatic-drug-therapies-among-patients-with-rheumatoid-arthritis-switching-from-another-biologic/