Session Information
Date: Monday, November 9, 2020
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The aim of this study was to compare quality of life (QoL) between chronic back pain (CBP) patients with and without a diagnosis of axial spondyloarthritis (axSpA), after two-years of protocolised follow-up.
Methods: Patients over 16 years of age referred to the rheumatology outpatient clinic with chronic back pain (≥ 3 months and < 2 years) starting before the age of 45, suspected of axSpA were included in the SPACE cohort. Follow-up was performed only in patients with at least two SpA features or one SpA feature with a positive likelihood ratio ≥6.4 (Rudwaleit, van der Heijde, Khan, Braun, & Sieper, 2004).
QoL was assessed using the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36). Age, sex and country weighted scale scores were calculated for each of the 8 subscales. Numeric scales ranged from 0 (worst health) to 100 (best health), after recoding and recalibration. The physical (PCS) and mental component summary (MCS) scores were calculated from the adjusted scores; and transformed to enable comparison to the general population mean of 50.
Additionally, the proportion of patients with an improvement or worsening of the PCS and MCS above the minimal clinically important difference (MCID) were assessed. We applied the commonly used MCID in clinical trials with bDMARDs in axSpA of 3 points for the PCS and MCS.
In this study we included patients with a diagnosis axSpA or no axSpA (CBP group), all with a level of confidence ≥7 (on a 0-10 scale) after locally read imaging, who had data available on the PCS and MCS at both timepoints.
Linear regression models were used to test the difference between groups at two-year follow-up for PCS and MCS scores. Baseline PCS and MCS scores and NSAID-use over time were tested as confounders.
Results: Patients with a diagnosis of axSpA were more frequently male and HLA-B27 positive and had more SpA features at baseline compared to the patients with CBP (Table 1). Age, symptom duration and NSAID-use were similar between groups.
In both groups the PCS significantly improved over two years. However, the PCS was significantly better in the group with an axSpA diagnosis compared to the CBP group at two-year follow-up, after correction for baseline PCS scores and NSAID-use over time (table 2). Despite the improvements over time, PCS scores were still well below the general population mean of 50 in both groups at two-year follow-up. MCS scores were not significantly different between groups at follow-up, and they were close to the general population mean.
In these regression models with baseline values and NSAID-use over time as covariates, axSpA was an independent predictor of better PCS scores (data not shown).
The majority of patients in both groups improved their PCS scores with more than the MCID over two-years of protocolised follow-up. In contrast, the proportion of patients who improved or worsened more than the MCID in MCS scores are similar. Also the percentage of patients with an improvement or worsening larger than the MCID for either PCS or MCS is similar in axSpA and CBP patients.
Conclusion: After two years of protocolised follow-up the physical functioning as assessed by the SF-36 PCS was better in patients with an axSpA diagnosis compared to patients with CBP.
 Table 1 Baseline characteristics of patients with an axSpA diagnosis and those with CBP.
Table 1 Baseline characteristics of patients with an axSpA diagnosis and those with CBP.
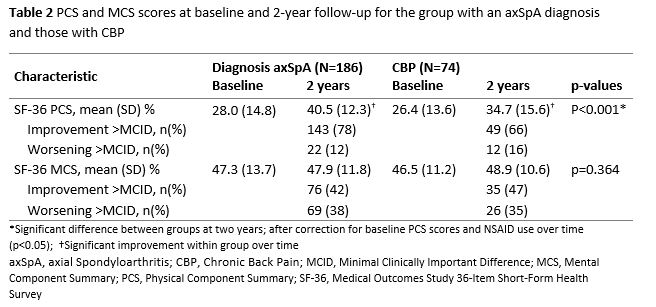 Table 2 PCS and MCS scores at baseline and 2-year follow-up for the group with an axSpA diagnosis and those with CBP
Table 2 PCS and MCS scores at baseline and 2-year follow-up for the group with an axSpA diagnosis and those with CBP
To cite this abstract in AMA style:
Boel A, van Lunteren M, Fagerli K, Exarchou S, Ramonda R, van de Sande M, van der Heijde D, van Gaalen F. Comparison of Quality of Life Between Chronic Back Pain Patients with and Without a Diagnosis of Axial Spondyloarthritis After 2-Year Protocolised Follow-up: Data from the Spondyloarthritis Caught Early Cohort [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparison-of-quality-of-life-between-chronic-back-pain-patients-with-and-without-a-diagnosis-of-axial-spondyloarthritis-after-2-year-protocolised-follow-up-data-from-the-spondyloarthritis-caught-ear/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-quality-of-life-between-chronic-back-pain-patients-with-and-without-a-diagnosis-of-axial-spondyloarthritis-after-2-year-protocolised-follow-up-data-from-the-spondyloarthritis-caught-ear/
