Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Patient self-reported medication histories may be prone to misclassification and recall bias. We aimed to assess the agreement between patient (Pt) and physician (MD) reported medication use in a cohort of RA patients.
Methods
Patients enrolled in the Ontario Best Practices Research Initiative (OBRI), a clinical registry of RA patients followed in routine care, were included. Following patient consent, data were extracted from physician charts using a structured questionnaire on patient demographics, comorbidities, disease activity and use of RA medications. Patients are assessed every 3 months through telephone interviews according to a standardized protocol to collect additional socio-economic characteristics, disease activities measures, and medication use. We examined the concordance of reporting medication names and the level of agreement between Pt and MD reported RA medications. RA medications (only DMARDs and BIOLOGICS) were categorized as (yes/no) for both self-reported and physician reported data and kappa statistics with 95% confidence intervals were computed at baseline and 12 month period to assess chance-corrected agreement between the two sources of data. Percent agreement was also calculated as a measure of agreement. In addition, dose reported for various drugs were compared at baseline and one year. Wilcoxon signed rank test was used to compare the mean difference of doses reported.
Results
Of the 2347 patients included in the study, 77% of patients were female with a mean (SD) age of 57.4 (12.9) years, and the majority (85%) were Caucasian. Patients had moderate disease activity according to both mean (SD) DAS28 scores 4.5 (1.5) and CDAI scores 21(14). At baseline, substantial agreement was found between Pt and MD reported medication use (kappa =0.78, 95% confidence interval (CI), 0.77-0.79); agreement=97.4%), and use of specific BIOLOGIC and DMARDS with agreement ranged from 88 to 100% (Figure, kappa range 0.50-0.85). The degree of agreement was lowest for methotrexate (kappa=88%). The MD reported mean dose was significantly higher than Pt except for MTX. At one year, the overall agreement of reported medication name increased with kappa=0.84 (95% CI 0.83-0.85) and there was no significant difference in reported dose between Pt and MD.
Conclusion
Similar level of agreement between patients and physicians suggest that differential misclassification is unlikely in the reporting of RA medication use. Furthermore, the accurate reporting of doses at one year suggests that patient’s ability to report their medications improves over time.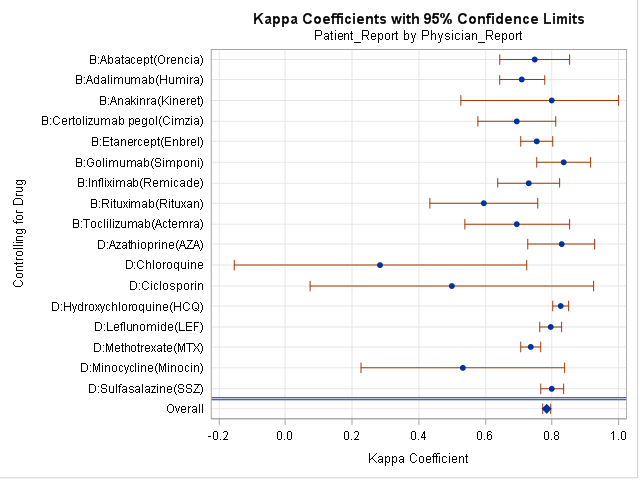
Disclosure:
B. Jacob,
None;
X. Li,
None;
A. Cesta,
None;
B. Kuriya,
None;
E. Keystone,
Abbott, Amgen, AstraZeneca, BMS, F. Hoffmann-La Roche, Janssen, Lilly, Novartis, Pfizer Sanofi-Aventis, UCB,
2,
Abbott Laboratories, AstraZeneca, Biotest, BMS, F. Hoffmann-La Roche, Genentech, Janssen, Lilly, Merck, Pfizer, UCB,
5,
Abbott, AstraZeneca, BMS Canada, F. Hoffmann-La Roche, Janssen, Pfizer, UCB, Amgen,
8;
C. Bombardier,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-patient-self-reported-and-physician-reported-rheumatoid-arthritis-medication-use-results-from-the-ontario-best-practices-research-initiative/
