Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate (MTX) is the mainstay first-line therapy for rheumatoid arthritis (RA), and it can be given orally or parenterally. Bioavailability of oral MTX may decrease at high doses, and parenteral administration could bypass this limitation. Practice guidelines recommend changing to biologics if response to MTX is suboptimal but these guidelines are based on studies comparing biologics to oral MTX. There is some evidence that parenteral MTX may be more efficacious than the oral form at equivalent doses. Also, studies suggest that the side effect profile of parenteral MTX may be better than oral MTX. We carried out a meta-analysis to compare the efficacy of oral versus parenteral MTX in RA.
Methods:
PubMed, Web of Science and Embase were systematically searched from inception to June 8th 2017 and reviewed following PRISMA 2009 guidelines. We also examined bibliographies of reviews and other articles. To be included, trials had to study adults with RA randomized to the same dose of either oral or parenteral MTX. Studies were selected and data extracted by two independent reviewers and at each stage the reviewers met to adjudicate discrepancies. The primary endpoint was ACR20 at 6 months. Other endpoints included ACR50, ACR70, SDAI, and DAS28 remission. One large trial allowed switching for poor response at 16 weeks and included only 24 week data, and trial authors reanalyzed data at 16 weeks. For another trial, data were reanalyzed to provide ACR20/50/70 rates. Intention-to-treat analyses were used when possible. Data from direct comparisons between oral and parenteral methotrexate were pooled and quantitatively analyzed using maximum likelihood random effects meta-analysis. Relative treatment effects were generated as odds ratio [OR] (OR>1 indicated a benefit for parenteral therapy). We also examined the mean difference in ACR20 rates between parenteral and oral MTX.
Results:
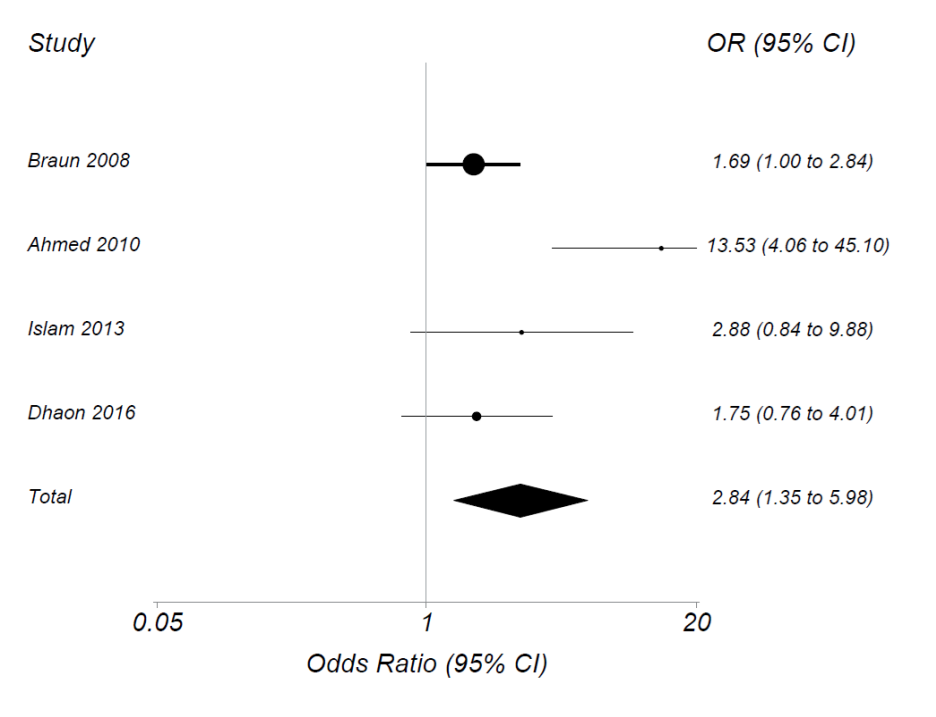
The search yielded 357 papers or abstracts. After review of titles or abstracts, we excluded 314. We then examined 43 full-text papers or abstracts and found 4 that met inclusion criteria with 703 patients randomized. Dose of MTX started at 15mg/week and increased to as high as 22.5mg/week. In each trial, ACR20 rates were higher for those randomized to parenteral than to oral MTX. The summary OR for achieving ACR20 using parenteral vs. oral MTX was 2.84 (95% CI 1.35, 5.98) (see figure). Those on parenteral had an 18.5% (95% CI 3.3%, 33.6%) greater absolute risk of attaining ACR20 than those on oral MTX. Similar results were seen for ACR50 and 70 and for SDAI and DAS.
Conclusion:
In this meta-analysis, parenteral MTX therapy had a significantly higher odds than oral MTX of achieving reduction in disease activity. We propose that parenteral MTX is more effective than oral MTX with a better safety profile; its widespread use may lead to better control of disease and a decrease in demand for biologic agents.
To cite this abstract in AMA style:
Janjua S, Bujor A, LaValley MP, Duran J, Braun J, Felson DT. Comparison of Oral Versus Parenteral Methotrexate in Rheumatoid Arthritis: A Meta-Analysis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparison-of-oral-versus-parenteral-methotrexate-in-rheumatoid-arthritis-a-meta-analysis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-oral-versus-parenteral-methotrexate-in-rheumatoid-arthritis-a-meta-analysis/