Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders I: Clinical Trials
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: The management of Takayasu’s arteritis (TAK) is challenging for lack of large-scale, high-quality clinical trials. Cyclophosphamide (CYC) is a conventional first-line immunosuppressant but is associated with reproductive toxicity and increased risk of malignancy. This study evaluated the efficacy and safety of mycophenolate mofetil (MMF) combined with methotrexate (MTX) compared to CYC followed by azathioprine (AZA) in treating active TAK.
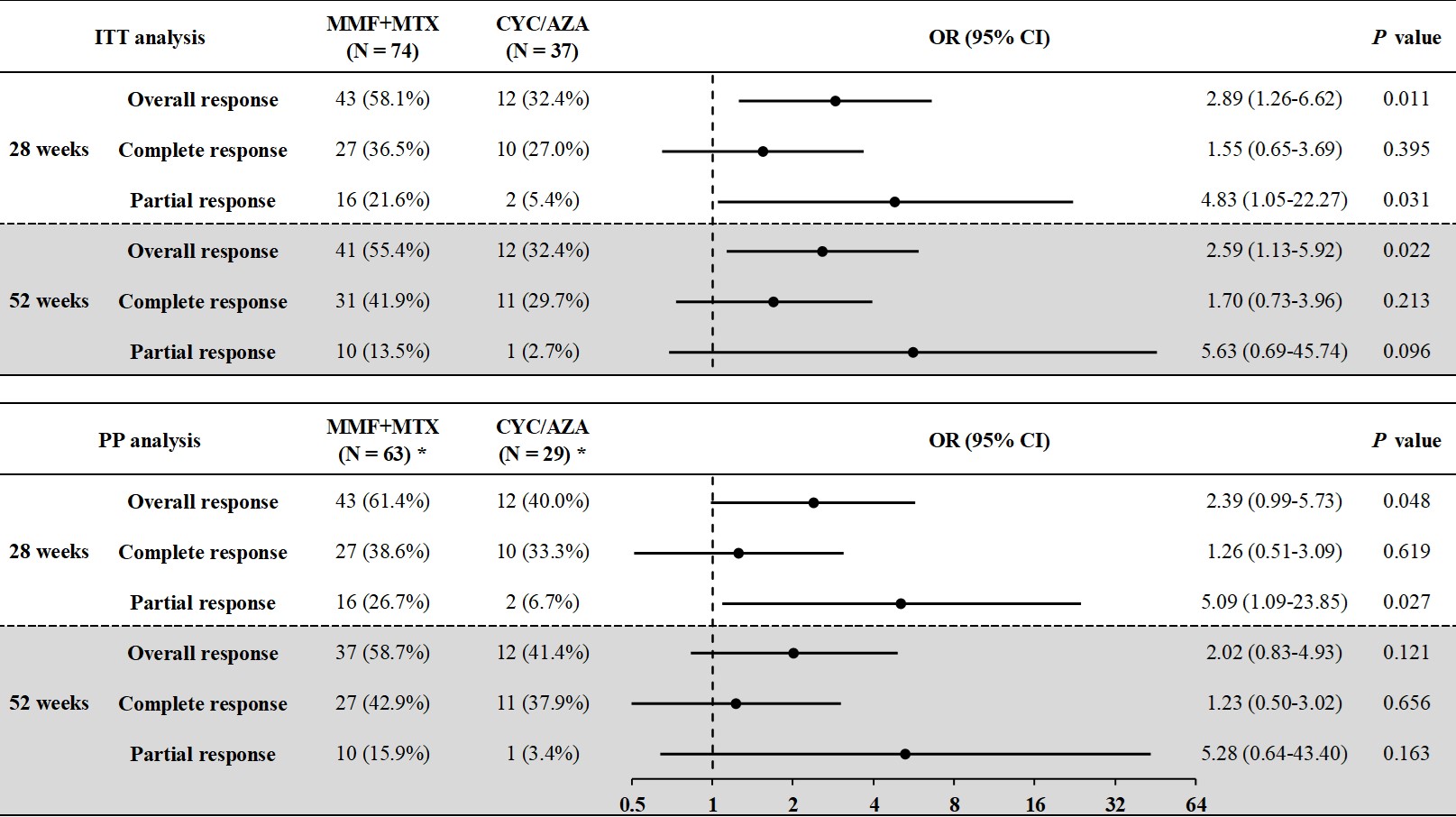
Methods: Patients aged 18 or older with active TAK were randomized 2:1 to receive MMF+MTX or CYC/AZA. All subjects received high-dose glucocorticoids (GCs) with a predefined tapering plan. MMF was dosed at 750mg or 1000mg twice daily based on body weight (< 50kg or ≥50kg), and MTX was given at 15mg weekly. CYC was administered intravenously at 15mg/kg for 10 infusions over 28 weeks, followed by oral AZA at 100mg daily. Patients were followed for 52 weeks to evaluate treatment efficacy and safety. The primary endpoint was the overall response rate at 52 weeks. Secondary endpoints included complete and partial response rates at 28 and 52 weeks. Complete response was defined as the resolution of active disease signs, normalization of ESR and CRP, no progression on imaging, and GCs dose < 15mg daily of prednisone or equivalent. Partial response was the same as complete response but with ESR and CRP below twice the upper normal limit or a 50% decrease from baseline. Overall response included both complete and partial response.
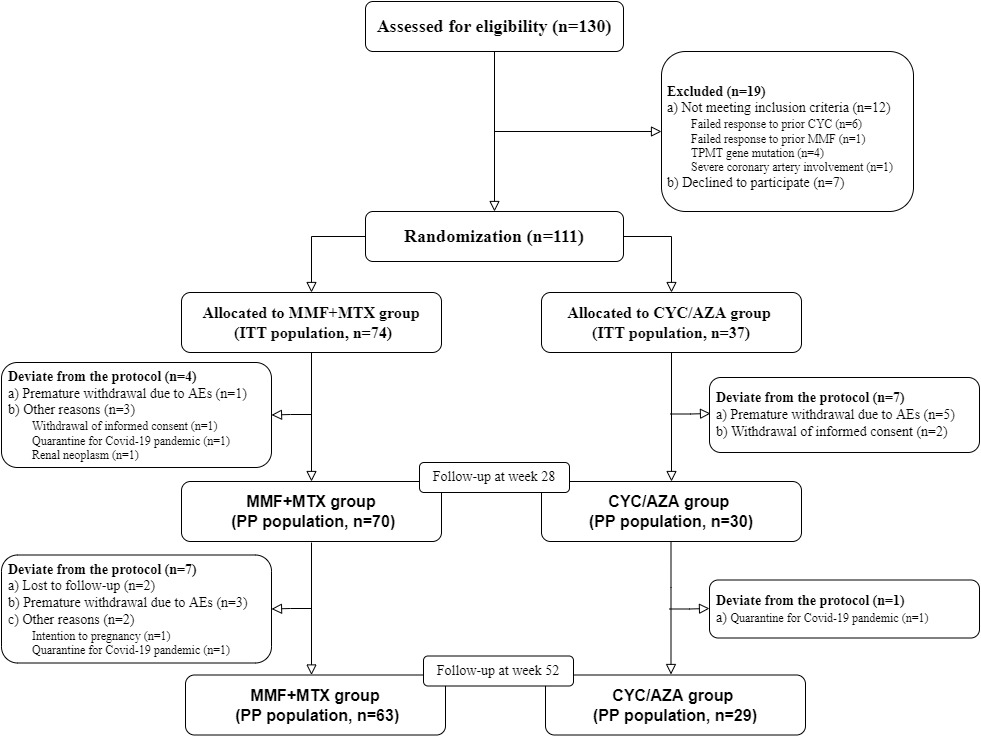
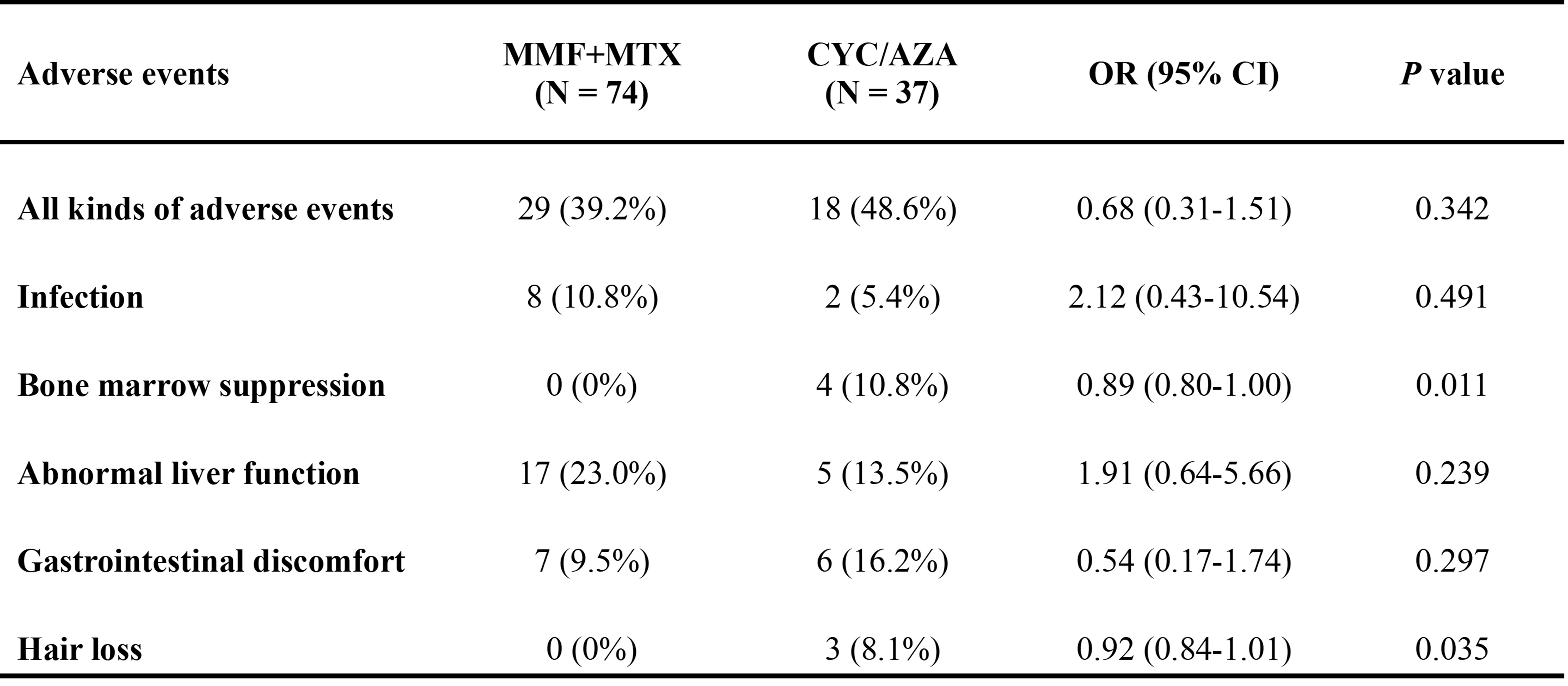
Results: A total of 111 patients were enrolled: 74 in the MMF+MTX group and 37 in the CYC/AZA group. Baseline demographic features, vascular lesions, inflammatory markers, and concurrent medications were comparable between the groups. Eleven patients in the MMF+MTX group and eight in the CYC/AZA group were excluded from the per-protocol (PP) analysis for deviation from the protocol. In the intent-to-treat (ITT) analysis, the overall response rates at 28 and 52 weeks were 58.1% and 55.4% in the MMF+MTX group respectively, significantly higher than 32.4% at both time points in the CYC/AZA group. Complete and partial response rates at 28 and 52 weeks were also higher in the MMF+MTX group. Similar results were observed in the PP analysis. Relapse occurred in 4 patients in the MMF+MTX group and 2 in the CYC/AZA group. The therapeutic benefit of MMF+MTX was more pronounced in patients without prior MTX use and those with diffuse artery involvement. Adverse events were reported in 29 (39.2%) patients in the MMF+MTX group and 18 (48.6%) patients in the CYC/AZA group. Apart from one case of neutropenia with fever in the CYC/AZA group, all adverse events were mild. Infections, abnormal liver function tests and gastrointestinal discomfort were the most common adverse events with similar frequency in both groups. Bone marrow suppression and hair loss occurred only in the CYC/AZA group.
Conclusion: MMF+MTX has superior efficacy in inducing and maintaining remission with a comparable safety profile to CYC/AZA in TAK patients over 52 weeks. These findings suggest that MMF combined with MTX could be the preferred treatment regimen for patients with active TAK compared to CYC followed by AZA.
* In the PP population at week 28, 70 patients in the MMF+MTX group and 30 patients in the CYC/AZA group were included.
Abbreviations: ITT, intention-to-treat; PP, per-protocol; OR, odds ratio; CI, confidence interval.
To cite this abstract in AMA style:
SUN X, Li J, Duan X, Zhang L, Yao D, Xue J, wu z, Zhao Y, Wu L, ZHANG H, LI M, Zeng X, Merkel P, Tian X. Comparison of Mycophenolate Mofetil Plus Methotrexate versus Cyclophosphamide with Sequential Azathioprine for Active Takayasu’s Arteritis: An Open-Label, Randomized-Controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparison-of-mycophenolate-mofetil-plus-methotrexate-versus-cyclophosphamide-with-sequential-azathioprine-for-active-takayasus-arteritis-an-open-label-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-mycophenolate-mofetil-plus-methotrexate-versus-cyclophosphamide-with-sequential-azathioprine-for-active-takayasus-arteritis-an-open-label-randomized-controlled-trial/