Session Information
Date: Monday, October 22, 2018
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Health activity tracker devices (e.g. Fitbit) are increasingly used component of medical evaluation. However, the validity and suitability of the data from such devices for research is not clear, specifically, methods are lacking to ascertain whether patients are consistently wearing the device and if the data is complete. We compared various methodologic approaches to describe the completeness of data capture in the context of a pilot study of patients with inflammatory arthritis.
Methods: We evaluated active & passively collected data from a trial of patients with gout who had a recent flare. All patients were given a Fitbit Charge HR2. Fitbit data (step count, sleep, heart rate [HR]) was evaluated in 1-minute increments. Minute-level HR data was compared with a gold standard for ‘Complete Wear’ as composite of step count, sleep, and HR, with imputation to 60-minute increments. Imputation of wear was performed for shorter intervals (1, 15, 30-minutes). Definitions for Complete of wear at a person-day level were evaluated using various parameters (e.g. ≥1200 minutes, or ≥800 minutes for days without sleep data). Variability in step count data was decomposed as between-person; within-person, between-day; and within-person, within-day (including error) based upon comparing sums of squares to total variance.
Results: At time of evaluation, 36 people contributed 4,534 person-days of observation. A total of 60% of person-days were considered Complete data with sleep, 14% as Complete data without sleep, and 26% with Partial Data. On days with Complete wear with sleep (gold standard), 13% of person-minutes were misclassified if only minute-level HR data was used, and step count & sleep data were ignored, while 8% were misclassified if only HR data was used, with imputation. Adding sleep and step count data, 12% of person-minutes were misclassified if minute-level data without imputation was used; 6% of person-minutes were misclassified if imputation to 60-minutes was applied, but the 1200/800 minute thresholds for Complete wear over a day were not required.
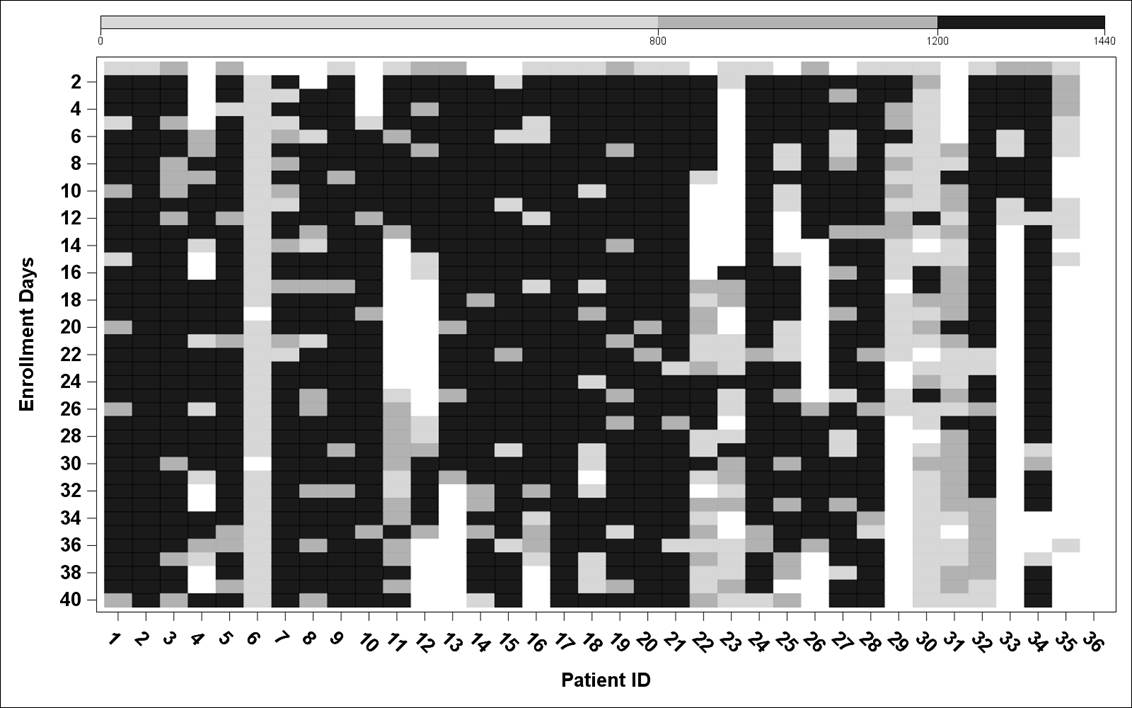
Imputation at 60-minute intervals yielded similar results to imputation at 1, 15, and 30-minute increments. There were intervals as long as 3+ hours where patients were wearing the device (based on gold standard) yet had no HR data. Among Complete wear days, variance in the step count data was much more related to between-person variability (36%) rather than within-person, between-day variability (<1%). With imputation at 60-minute increments, wear patterns are shown in the heat map (Figure).
Conclusion: Fitbit and other health tracker data appear useful as part of arthritis research, but several methodologic issues must be considered to maximize validity and interpretation.
Figure: Heat Map – Data of Complete Wear with Sleep (>1200 minutes) [Black], Complete Wear without Sleep [Dark Gray], and Incomplete Wear [Light Gray]
To cite this abstract in AMA style:
Curtis JR, Yang S, Chen L, Elmagboul N, Redden DT, Mudano AS, Foster PJ, Cooper F, Mikuls TR, Owensby JK, Saag K. Comparison of Methodologic Approaches to Maximize the Validity of Fitbit Device Data for Arthritis-Related Research Purposes [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/comparison-of-methodologic-approaches-to-maximize-the-validity-of-fitbit-device-data-for-arthritis-related-research-purposes/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-methodologic-approaches-to-maximize-the-validity-of-fitbit-device-data-for-arthritis-related-research-purposes/