Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: The risk and cost of infection-related hospitalizations in tumor necrosis factor inhibitors (TNFi)-experienced patients with rheumatoid arthritis (RA) receiving subsequent targeted disease-modifying antirheumatic drug (tDMARD) is unknown in the Medicare population. This study compared the risk and cost of infection-related hospitalization costs between TNFi-experienced patients receiving abatacept, TNFi and other non-TNFi.
Methods: A retrospective cohort study using 100% Medicare fee-for-service (FFS) claims (Parts A/B/D) was designed to identify TNFi-experienced patients with RA who initiated a subsequent treatment with abatacept, TNFi (adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab) or other non-TNFi (anakinra, sarilumab, rituximab, tocilizumab, tofacitinib) between January 2010-December 2017. The date of the new tDMARD therapy initiation was the index date. Patients were included if age 65+, ≥2 claims with a diagnosis code for RA, continuous enrollment 12-months pre- and post-index date, and no evidence of cancer and other auto-immune conditions. Follow-up ended at the date of disenrollment, death, end of study period, or end of index treatment, whichever occurred first. Infections included pneumonia, bacterial respiratory, sepsis, skin and soft tissue, joint or genitourinary. A two-part generalized linear model controlling for baseline demographics, comorbidities, infections, healthcare resource use and costs was used to examine differences in per-patient per month (PPPM) Medicare payments (2019 USD). A Cox regression model was used to compare the risk of infections.
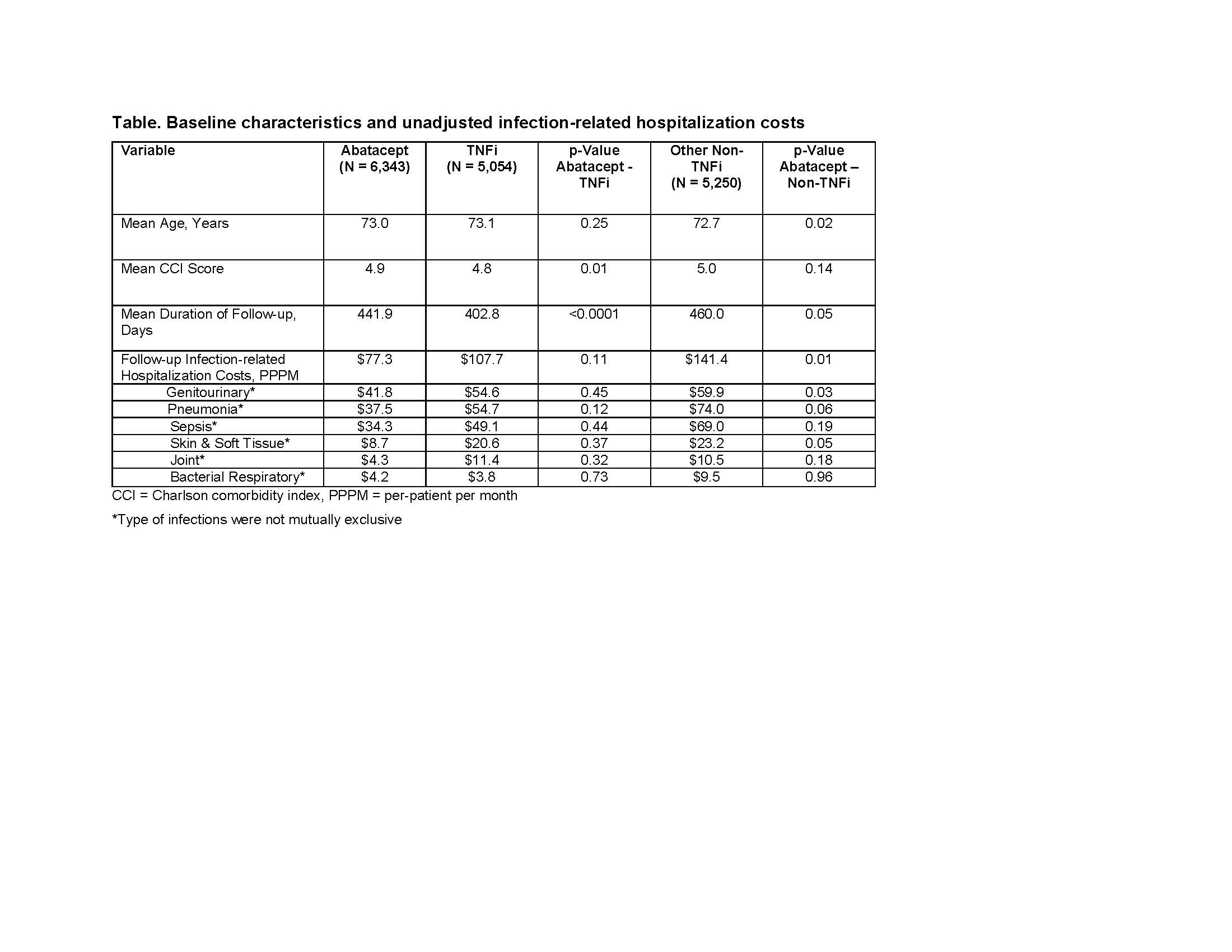
Results: Of the study population, 6,343 patients were treated with abatacept, 5,054 with TNFi, and 5,250 with non-TNFi. At baseline, abatacept group had the highest proportion of patients with infection-related hospitalization (9.8% vs. 7.1% vs. 9.7%, P< 0.05). However, the risk of infection-related hospitalizations during follow-up was lower in patients treated with abatacept compared with TNFi (7.5% vs. 8.1%, P=0.21) and other non-TNFis. (7.5% vs. 10.7%, P< 0.0001). After adjusting for differences in patient characteristics, Cox regression analysis showed that the risk for an infection-related hospitalization was significantly higher for RA patients treated with TNFi’s (HR: 1.48; 95% CI: 1.26-1.75, P< 0.0001) and other non-TNFi’s (HR: 1.46; CI: 1.28-1.66, P< 0.0001) than in those treated with abatacept. During follow-up, after controlling for potential confounding variables, infection-related hospitalization PPPM cost was significantly higher in patients treated with a TNFi’s (difference: $93; 95% CI: $51-$134) and other non-TNFi’s (difference: $84; 95% CI: $52-$116) than in those treated with abatacept.
Conclusion: Medicare FFS beneficiaries with RA who initiated abatacept following the exposure of TNFi, had lower infection-related hospitalization costs compared to patients who switched to a different TNFi or other non-TNFi.
To cite this abstract in AMA style:
Patel V, Pulungan Z, Shah A, Kambhampati M, Lobo F, Petrilla A. Comparison of Infection-Related Hospitalization Risk and Cost in TNFi-Experienced Medicare Beneficiaries with Rheumatoid Arthritis Treated with Abatacept or Other Targeted Disease-Modifying Anti-Rheumatic Drugs [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparison-of-infection-related-hospitalization-risk-and-cost-in-tnfi-experienced-medicare-beneficiaries-with-rheumatoid-arthritis-treated-with-abatacept-or-other-targeted-disease-modifying-anti-rheum/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-infection-related-hospitalization-risk-and-cost-in-tnfi-experienced-medicare-beneficiaries-with-rheumatoid-arthritis-treated-with-abatacept-or-other-targeted-disease-modifying-anti-rheum/