Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Session Type: Poster Breakout Session
Session Time: 4:30PM-5:00PM
Background/Purpose: The use of intra-articular corticosteroid (IAC) injections for Juvenile Idiopathic Arthritis (JIA) was extrapolated from its use in adult inflammatory joint diseases to achieve rapid resolution of synovitis and to provide patient pain relief. In JIA, studies have reported long term benefits of IAC injections, including long term reduction in pain and inflammation, improvement and prevention of limb length discrepancy, and resolution of joint pannus.
Among available steroid formulations for IAC injections, lower solubility agents have slower absorption and longer duration of action, leading to higher efficacy. The most used formulations in Europe and North America are triamcinolone acetonide (TA) and triamcinolone hexacetonide (TH). Several studies have shown TH to be superior to TA in prolonging clinical remission in patients with JIA. However, commercial production of TH in the United States was halted in 2015. Without FDA approval for its use in patients with JIA, TH is not covered by American insurance companies. Our objective was to compare the efficacy of TH to the current standard of TA in maintaining clinical remission for patients with JIA. Secondary outcomes were to assess the yield of IAC injections in different joint locations, to assess the yield in patients with/without disease modifying risk factors (+ANA, HLA-B27, RF), and to assess the impact on the use of adjunctive systemic therapies.
Methods: In this retrospective chart review, EMR CPT codes identified JIA patients who received an IAC injection (TA or TH) at a single tertiary center between September 1, 2018 and September 1, 2019. Exclusion criteria included patients who have received in IAC injection within 3 months prior and patients with a clinically significant adverse reaction to corticosteroids. Medical records were reviewed for patient demographics and clinical course.
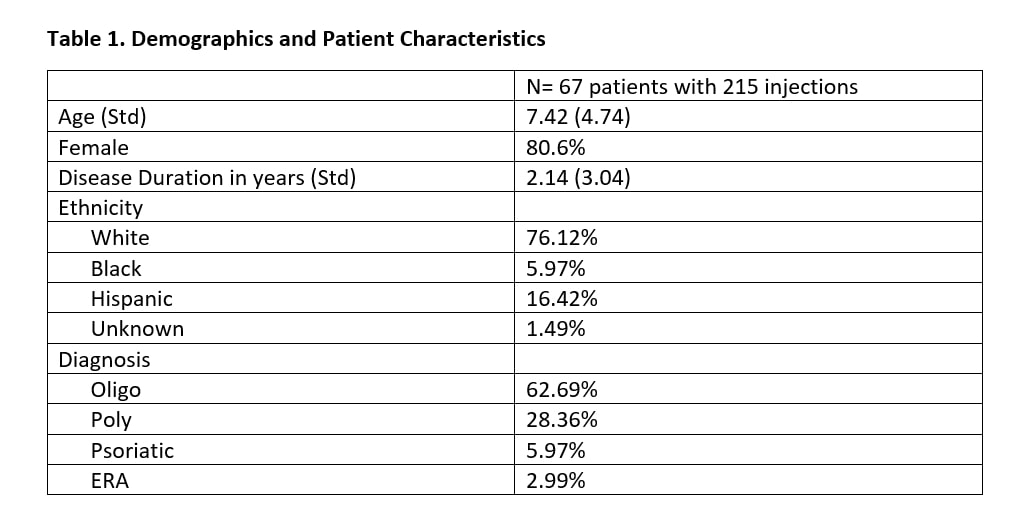
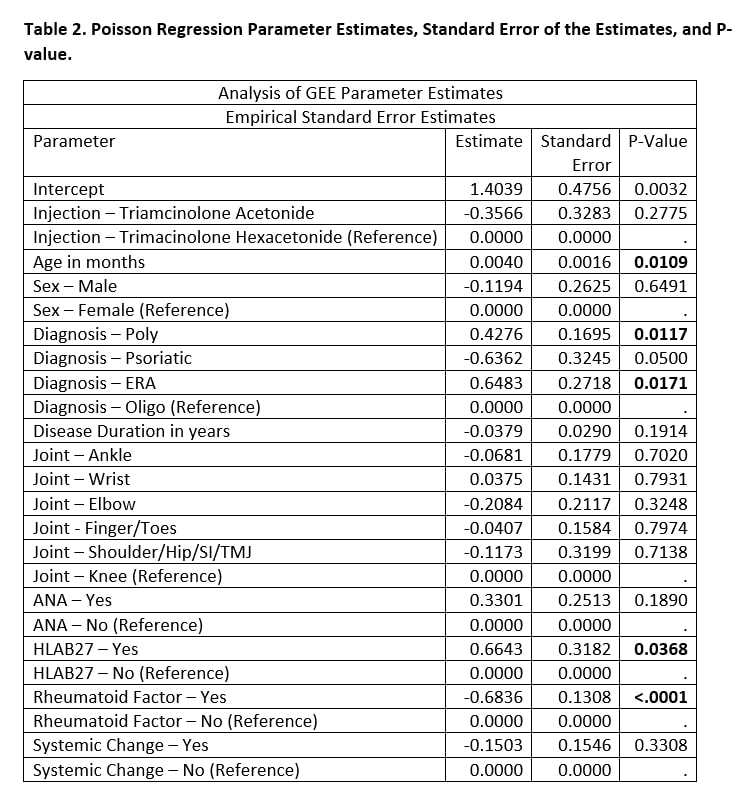
Results: A total of 215 joints were injected from 67 unique patients [Table 1]. Joints injected with TA had a median of 5 months in clinical remission, versus TH joints had a median of 10 months in clinical remission (Mann-Whitney U test p-value < 0.0001). Poisson regression model with generalized estimating equations found several statistically significant co-variates (p-value < 0.05), including age, poly-articular status, HLA-B27 status, and rheumatoid factor status [Table 2]. The log count of remission months decreased by 0.3566 for those that received TA when adjusting for the other co-variates in the model (p-value = 0.2775).
Conclusion: Joints injected with TH had double the median number of months in clinical remission compared to TA. Age, poly-articular status, HLA-B27 status, and rheumatoid factor status had statistically significant impact on log count of clinical remission months. Joint type was not a statistically significant co-variate. There was no statistically significant impact on the use of adjunctive systemic therapies.
To cite this abstract in AMA style:
Chun A, Muhammad L, De Ranieri D. Comparison of Efficacy Between Triamcinolone Acetonide and Hexacetonide Intra-articular Treatment for Clinical Remission in Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/comparison-of-efficacy-between-triamcinolone-acetonide-and-hexacetonide-intra-articular-treatment-for-clinical-remission-in-juvenile-idiopathic-arthritis/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-efficacy-between-triamcinolone-acetonide-and-hexacetonide-intra-articular-treatment-for-clinical-remission-in-juvenile-idiopathic-arthritis/