Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Dual-immunosuppressive regimens consisting of high-dose glucocorticoids and higher doses ( >2 g/day) of mycophenolate mofetil (MMF) are still frequently used for the initial management of active lupus nephritis (LN) despite the known serious and dose-dependent safety risks associated with both of these agents.
To understand the safety and efficacy of a voclosporin-based, MMF and glucocorticoid-sparing, triple immunosuppressive regimen as an initial approach to treatment in active LN compared to more conventional regimens, we compared and analyzed safety and efficacy data in propensity-matched patients from the ALMS and AURORA 1. We hypothesized that a voclosporin-based, triple immunotherapy approach would reduce patient exposure to the toxicities associated with glucocorticoids and MMF, resulting in an improved safety profile without compromising efficacy.
Methods: Both studies enrolled patients with active LN. In ALMS, MMF was dosed to a target of 3 g/day with oral glucocorticoids initiated at a maximum dose of 60 mg/day and tapered every 2 weeks to 10 mg/day. In AURORA 1, patients received voclosporin 23.7 mg BID in combination with MMF (target dose 2 g/day) and oral glucocorticoids (starting dose of 25 mg/day tapered to 2.5 mg/day by Week 16). Propensity score matching was used to generate two groups of matched patients based on a set of demographic and disease characteristics. Safety and efficacy outcomes were assessed at 3 and 6 months.
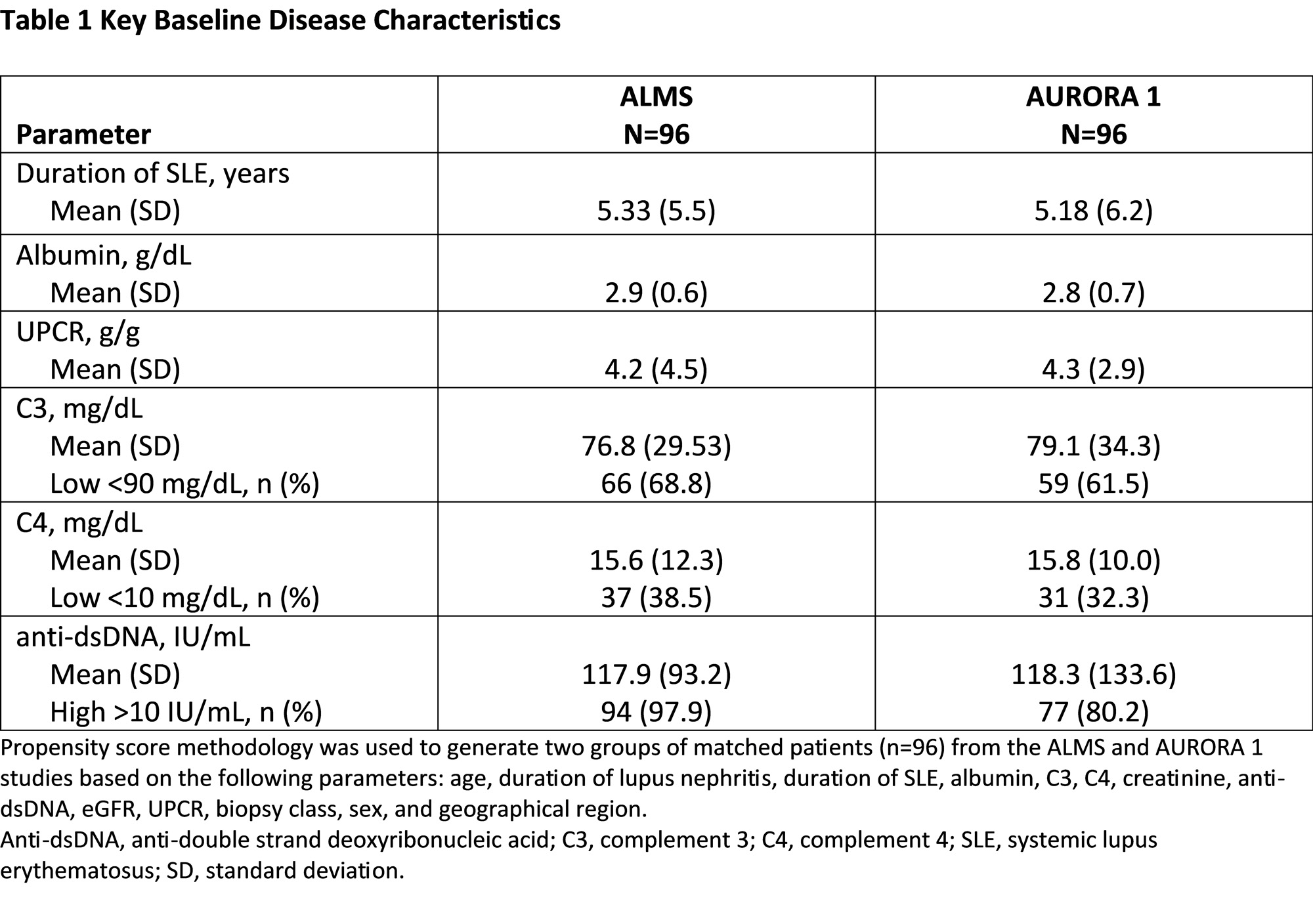
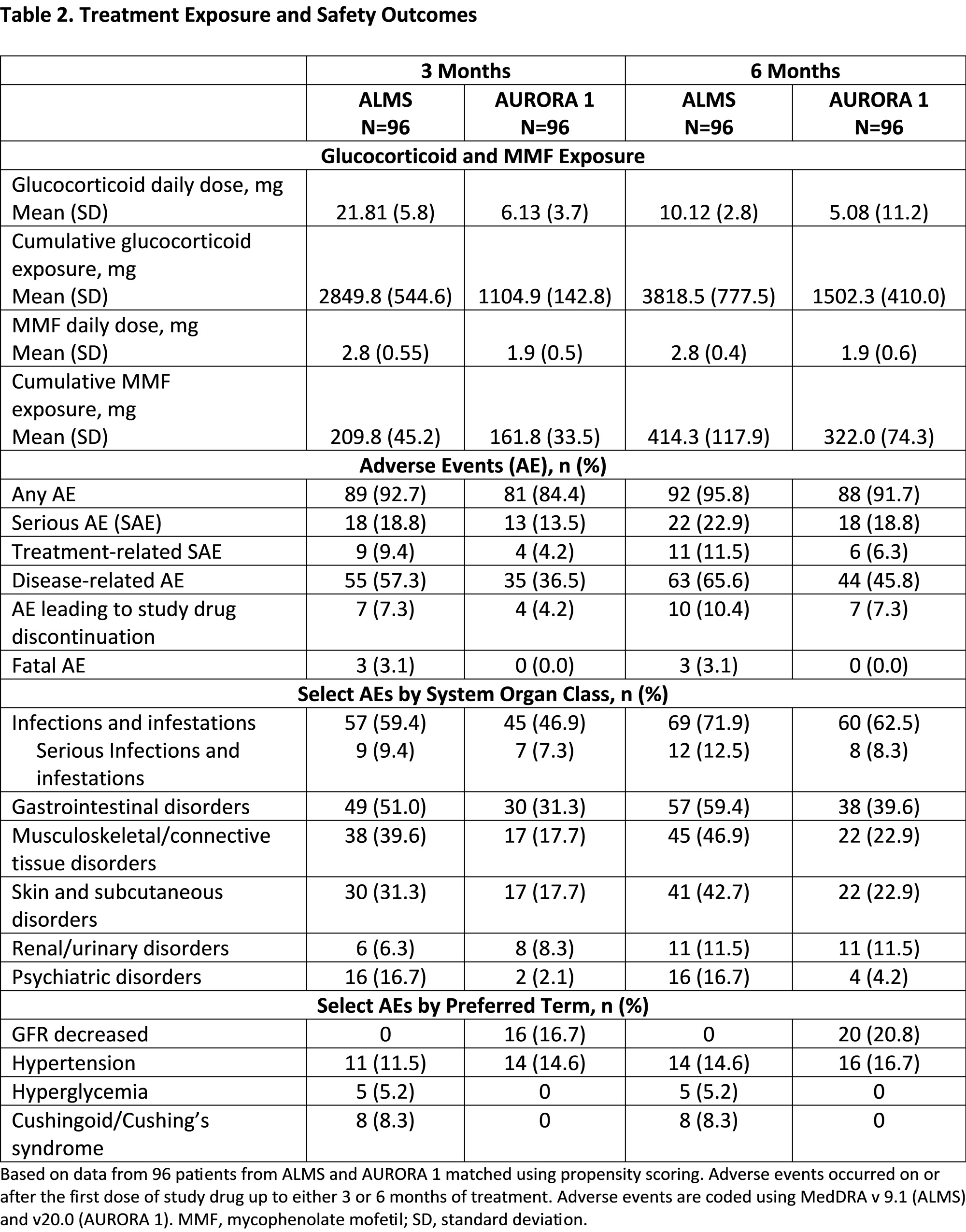
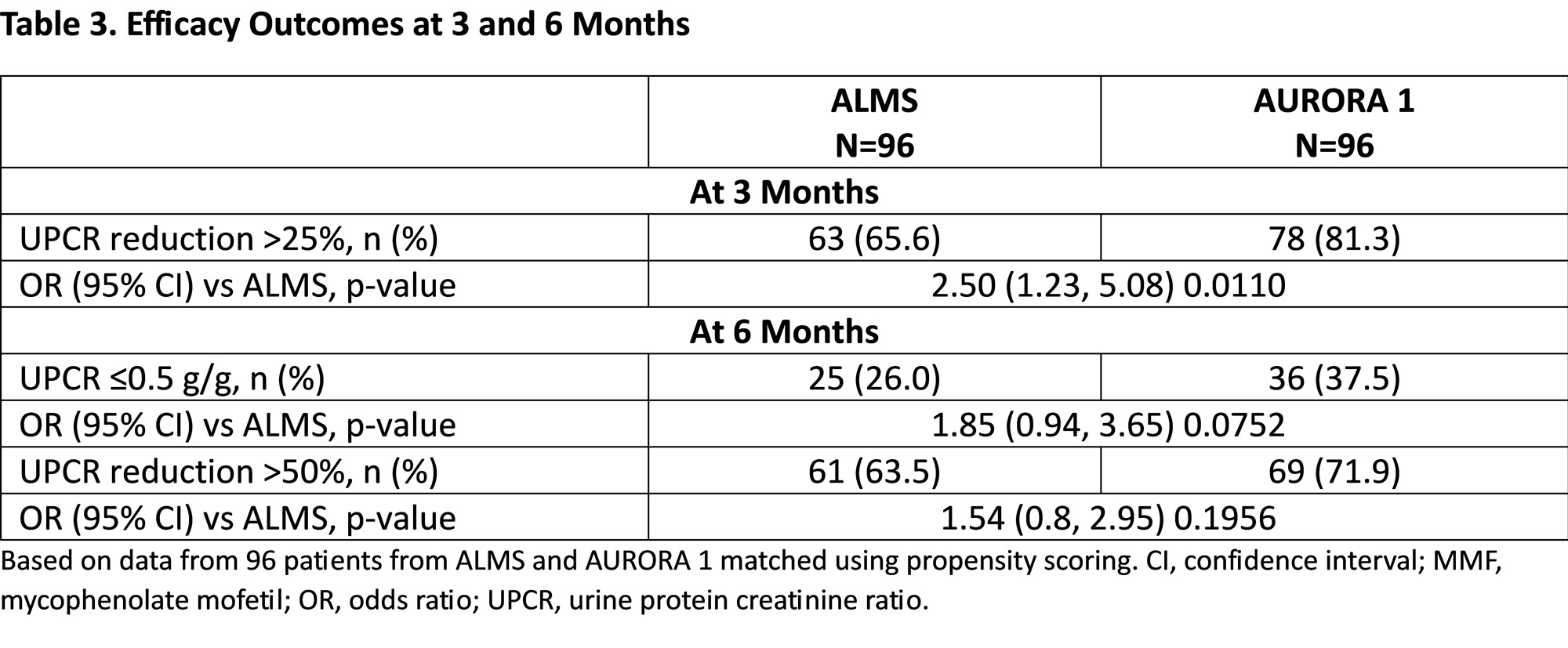
Results: Propensity matching identified 96 pairs of patients with similar demographics and baseline disease characteristics (Table 1). At 3 and 6 months, MMF and glucocorticoid exposure was more than two-fold higher in ALMS than AURORA 1. Overall, fewer adverse events were observed in AURORA 1 across the majority of organ systems, including gastrointestinal, skin and subcutaneous tissues, endocrine, and psychiatric disorders, although more patients in AURORA 1 were reported to experience GFR decrease (Table 2). The incidence of serious adverse events was similar in both groups at 3 and 6 months. In the first 3 months, significantly more patients in AURORA 1 achieved >25% UPCR reduction from baseline (p=0.011; Table 3); the proportions of patients achieving UPCR ≤0.5 g/g and >50% UPCR reduction from baseline were numerically greater in the voclosporin arm; the differences were not statistically significant.
Conclusion: Patients treated with voclosporin in combination with low-dose glucocorticoids and lower-dose MMF demonstrated an improved safety profile and earlier reductions in proteinuria compared to patients treated with high-dose glucocorticoids and higher doses of MMF. These findings affirm the KDIGO 2023 recommendation that a voclosporin-based, triple-immunotherapy regimen should be considered as an initial therapy in patients with active LN.
To cite this abstract in AMA style:
Dall'Era M, Yap E, Truman M, Hodge L, Solomons N. Comparison of Dual-immunosuppressive Therapy with a Voclosporin-based, Triple-immunosuppressive Regimen for Lupus Nephritis in the ALMS and AURORA 1 Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparison-of-dual-immunosuppressive-therapy-with-a-voclosporin-based-triple-immunosuppressive-regimen-for-lupus-nephritis-in-the-alms-and-aurora-1-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-dual-immunosuppressive-therapy-with-a-voclosporin-based-triple-immunosuppressive-regimen-for-lupus-nephritis-in-the-alms-and-aurora-1-studies/