Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Drug persistence represents long-term therapeutic performance in real clinical setting and tuberculosis (TB) is one of the most important adverse events following biological treatments in rheumatoid arthritis (RA). We aimed to compare persistence and incidence of tuberculosis between tumor necrosis factor-alpha (TNF-α) inhibitors and tocilizumab in Korean patients with (RA) using the nationwide claim database.
Methods: In this retrospective cohort study, patients with RA who started TNF-α inhibitors such as adalimumab, etanercept, infliximab and golimumab or tocilizumab as the first-line biological therapy between Jan 2014 and Dec 2017 on Korean Health Insurance Review and Assessment Service database were analyzed and were followed up to Dec 2019. Drug persistence was defined as the duration from the initiation to the first discontinuation including stopping and switching to other biological therapy and occurrence of TB was defined as the prescription of more than 2 anti-TB medications after the initiation of TNF-α inhibitors and tocilizumab.
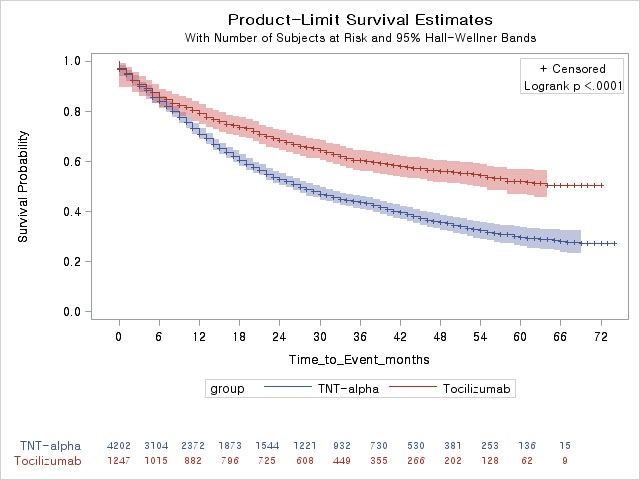
Results: A total of 5,449 RA patients were analyzed in this study. Mean age was 53.4 years and 4,423 (81.2%) patients were female. TNF-α inhibitors and tocilizumab were prescribed for 4,202 (adalimumab, 1,413; etanercept, 1,100; infliximab, 769; golimumab 920) and 1,247 RA patients, respectively. During the study period, 2,090 (49.7%) and 477 (38.3%) RA patients discontinued TNF-α inhibitors and tocilizumab, respectively. Drug persistence was significantly lower in TNF-α inhibitors group than tocilizumab group (log-rank test, p< 0.001) as shown in Figure 1. In multivariable Cox regression model, TNF-α inhibitors was significantly associated with a higher risk of discontinuation compared with tocilizumab (HR=1.63, 95% CI=1.47-1.81, p< 0.001). In subgroup analyses, adalimumab (HR=1.73, 95% CI=1.54-1.95, p< 0.001), etanercept (HR=1.87, 95% CI=1.66-2.11, p< 0.001) and golimumab (HR=1.57, 95% CI=1.37-1.79, p< 0.001) but not infliximab (HR=1.23, 95% CI=1.06-1.42, p=0.054) showed a significantly higher risk of discontinuation compared with tocilizumab after adjusting confounding factors. Forty-two RA patients developed TB (TNF-α inhibitors; 33 and tocilizumab; 9) during the follow-up period and there was no significant difference in the incidence of TB between TNF-α inhibitors and tocilizumab treatment groups (log-rank test, p=0.386).
Conclusion: Our study revealed a better long-term persistence of tocilizumab compared with TNF-α inhibitors in patients in RA in real clinical setting. No difference in TB incidence between TNF-α inhibitors and tocilizumab therapy was found.
To cite this abstract in AMA style:
Song B, Moon D, Kim A, Lee S. Comparison of Drug Persistence and Incidence of Tuberculosis Between Tumor Necrosis Factor-α Inhibitors and Tocilizumab as the First-line Biological Treatment in Patients with Rheumatoid Arthritis Using the Korean Health Insurance Review and Assessment Service Database [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/comparison-of-drug-persistence-and-incidence-of-tuberculosis-between-tumor-necrosis-factor-%ce%b1-inhibitors-and-tocilizumab-as-the-first-line-biological-treatment-in-patients-with-rheumatoid-arthriti/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-drug-persistence-and-incidence-of-tuberculosis-between-tumor-necrosis-factor-%ce%b1-inhibitors-and-tocilizumab-as-the-first-line-biological-treatment-in-patients-with-rheumatoid-arthriti/