Session Information
Date: Monday, November 6, 2017
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Remission in SLE is a desirable target, however there is no gold standard for the definition of remission. An international task force agreed on various definitions of remission in SLE (DORIS) [1]. Our aim was to apply these definitions on a clinical trial.
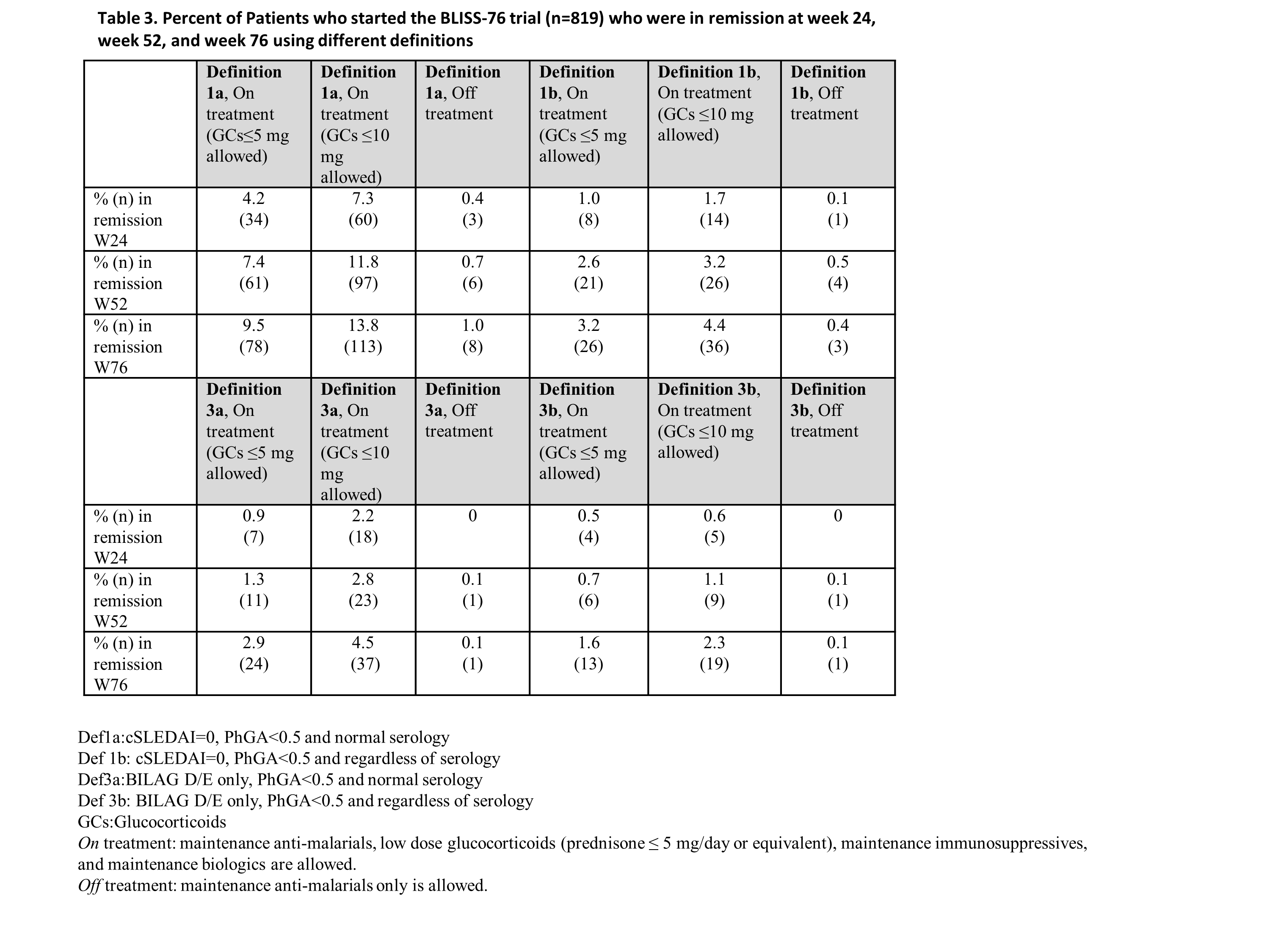
Methods: This is a post-hoc analysis of a prospective randomized controlled trial (RCT) in SLE, the BLISS-76 clinical trial [2]. We have applied two DORIS definitions (Table 1) at three time points, week (wk) 24, 52 and 76. The patient could be in remission on or off treatment. Remission on treatment allowed maintenance anti-malarials, low dose glucocorticoids (GCs) (prednisone ≤ 5 mg/day or equivalent), maintenance immunosuppressives and maintenance biologics. Remission off treatment allowed maintenance anti-malarials only. Additionally, we applied each definition where the remission on treatment allowed a GC dose ≤ 10 mg/day (not a part of the original DORIS-definitions).
Results: There were 819 patients enrolled in BLISS-76. The baseline characteristics are shown in Table 2. The proportions of patients that fulfilled remission according to the above definitions are shown in Table 3. The highest point prevalence (9.5%) was when definition 1a on treatment was applied at wk 76. As expected, even more patients fulfilled definition 1a when a GC dose ≤ 10 mg/day was allowed at wk 76 (13.8%). More patients fulfilled the remission criteria when clinical SLEDAI was used compared to when BILAG was used. When serology (anti-DNA antibodies and complement) was taken into consideration (definitions 1b and 3b), less patients fulfilled remission. Very low numbers of patients (≤1%) fulfilled remission off treatment.
Conclusion: Overall, few patients fulfilled remission according to these definitions. More patients fulfilled the definitions when serology was excluded and when a higher dose of GCs was allowed.
References
1. van Vollenhoven R, et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Annals of the rheumatic diseases. 2016.
2. Furie R, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918-30.
To cite this abstract in AMA style:
Emamikia S, Gentline C, Arkema EV, Arnaud L, Chatzidionysiou K, van Vollenhoven RF. Comparison of Different Definitions of Remission in Systemic Lupus Erythematosus (SLE)– a Study Based on the BLISS-76 Clinical Trial [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/comparison-of-different-definitions-of-remission-in-systemic-lupus-erythematosus-sle-a-study-based-on-the-bliss-76-clinical-trial/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-different-definitions-of-remission-in-systemic-lupus-erythematosus-sle-a-study-based-on-the-bliss-76-clinical-trial/