Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Sodium-glucose co-transporter 2 inhibitors (SGLT2i), originally approved as oral hypoglycemic agents for type 2 diabetes (T2D), have been shown to reduce progression to end-stage renal disease (ESRD), as well as heart failure and overall mortality. Those with systemic lupus erythematosus (SLE) were excluded from trials and thus the potential benefits for these patients are unknown. We conducted a simulated clinical trial comparing renal, cardiovascular, infectious, and mortality outcomes among patients with both SLE and T2D starting SGLT2i vs. dipeptidyl peptidase 4 inhibitors (DPP4i) in a large real-world administrative dataset.
Methods: We identified a cohort of patients with both SLE and T2D, age ≥ 18 years within administrative data from 92 hospitals across the United States (TriNetX) using billing claim algorithms. All patients initiated either an SGLT2i or DPP4i between 2014 and 2023. SGLT2i recipients were 1:1 propensity score (PS) matched to DPP4i recipients on demographic and clinical factors, also identified by billing codes. Cox models estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for the endpoints: 1) acute kidney injury (AKI), chronic kidney disease (CKD), end-stage renal disease (ESRD), 2) heart failure, myocardial infarction, stroke, 3) infections, specifically urinary tract and genital infections, severe sepsis, 4) emergency visits, hospitalizations and all-cause mortality.
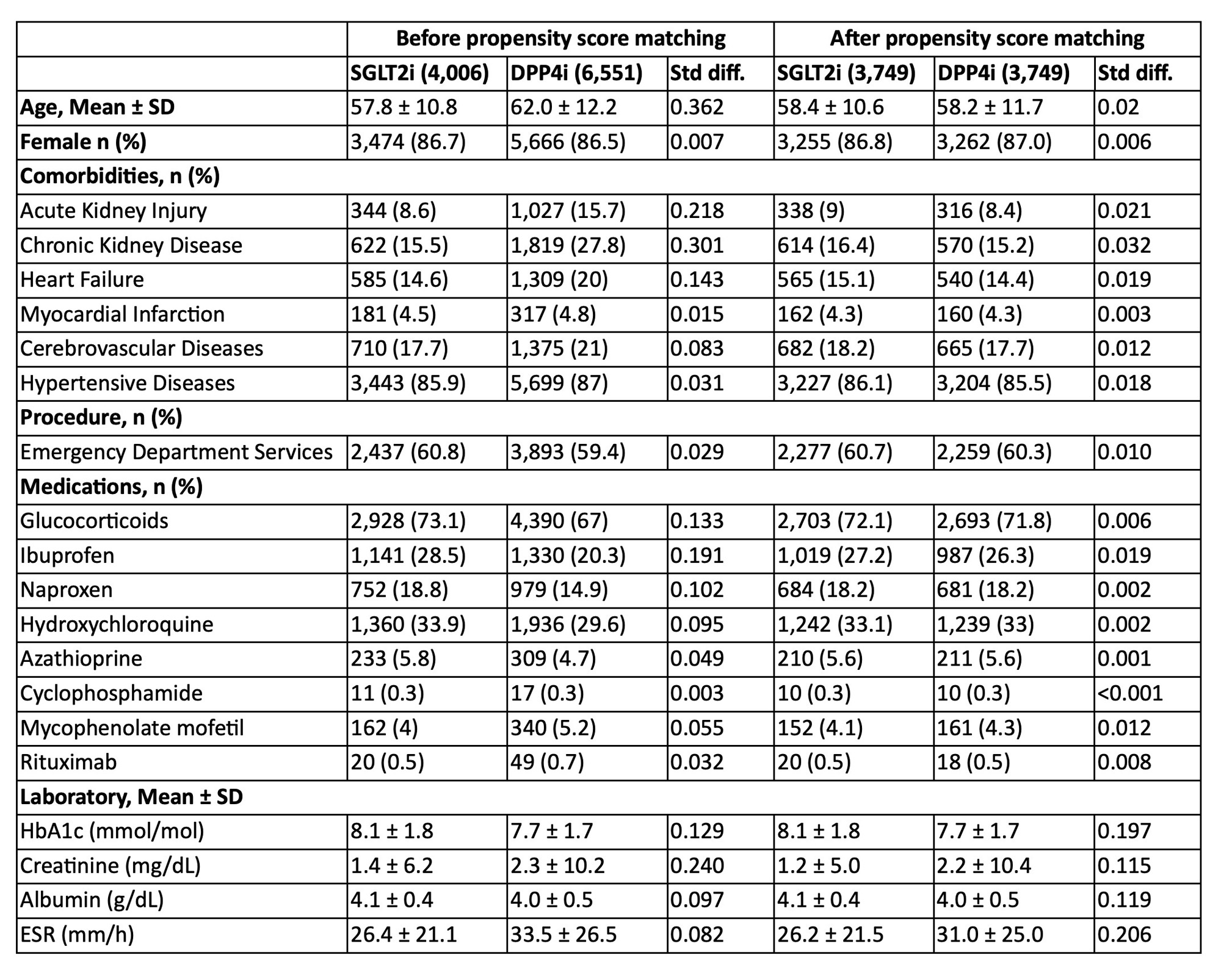
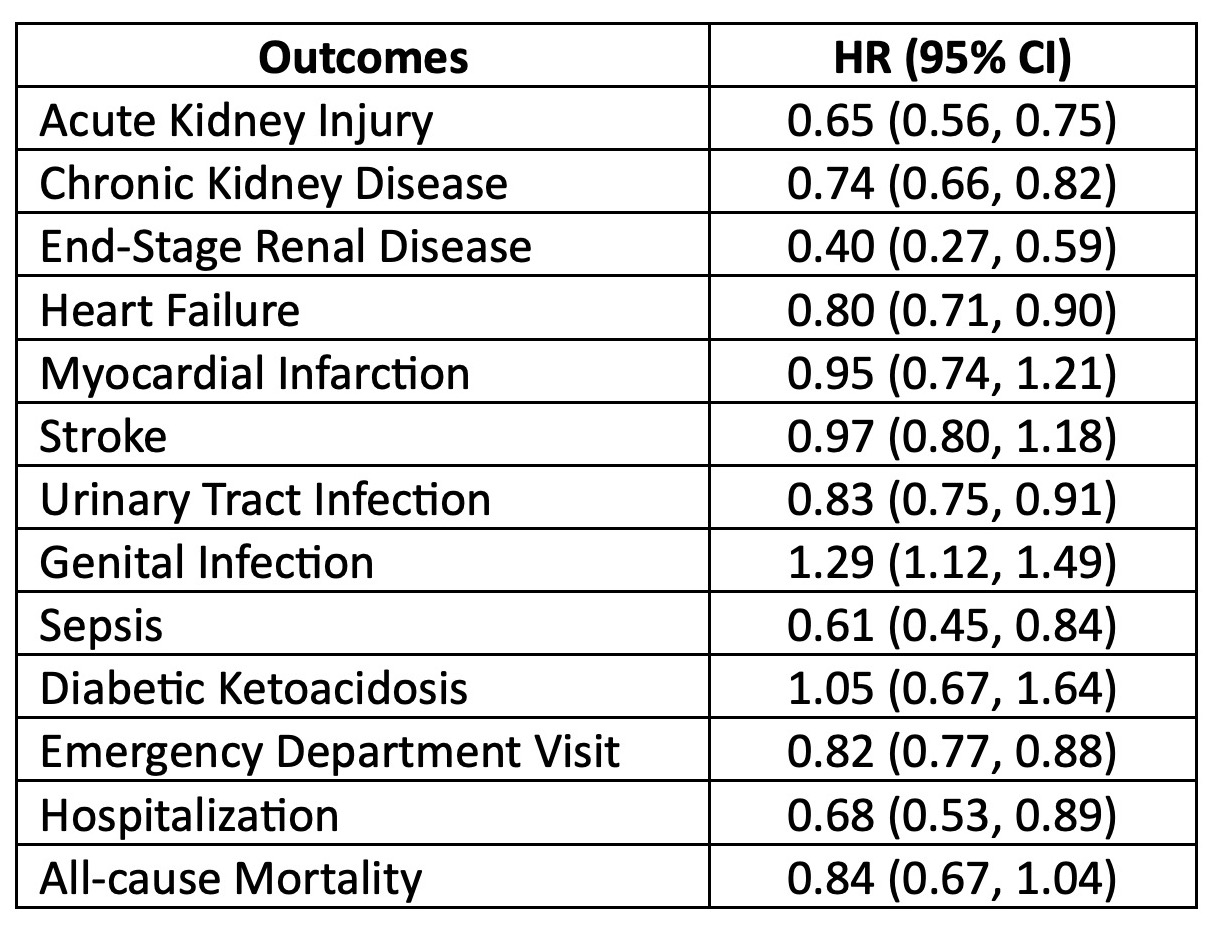
Results: Table 1 shows the SLE/T2D populations before and after PS-matching. Of the 3,749 PS-matched patients starting SGLT2i and 3,749 starting DPP4i, mean age was 58.3 (±11.2) years; 86.9% were females. In follow-up over 7.95 (±14.5) mean years, SGLT2i vs DPP4i recipients had significantly reduced risks of developing AKI (HR 0.65; 0.56-0.75), CKD (HR 0.74; 0.66-0.82), ESRD (HR 0.40; 0.27-0.59), and heart failure (HR 0.80; 0.71-0.90) (Table 2). SGLT2i use was also associated with decreased future emergency visits (HR 0.82; 0.77-0.88) and hospitalization (HR 0.68; 0.53-0.89). Risk of genital infection was elevated (HR 1.29, 1.12-1.49), but urinary tract infections (HR 0.90; 0.75-0.91) and severe sepsis (HR 0.61; 0.45-0.84) were reduced. We found no significant differences in all-cause mortality, diabetic ketoacidosis, or lower limb amputation between SGLT2i versus DPP4i use (Table 2).
Conclusion: In this real-world head-to-head simulated randomized trial in a large population of patients with SLE and T2D from 2014-2023, SGLT2i use compared to DPP4i use was associated with significantly reduced risks of developing AKI, CKD, ESRD, hospitalization, and heart failure. Genital infection risk was elevated, but risks of emergency visits, hospitalizations, urinary tract infections, and severe sepsis were also reduced for SGLT2i users. Overall mortality was not different. Thus, SGLT2i appear safe and effective for those with SLE and T2D. Given high risks of cardiovascular and renal disease in patients with SLE, further trials and studies should investigate whether SGLT2i should be used for patients with SLE without T2D.
To cite this abstract in AMA style:
Lo J, Huang P, Tsokos G, Kyttaris V, Costenbader K, Ma K. Comparing Safety and Efficacy of Sodium-Glucose Co-Transporter 2 Inhibitors versus Dipeptidyl Peptidase 4 Inhibitors in Patients with Systemic Lupus Erythematosus and Comorbid Type 2 Diabetes Mellitus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparing-safety-and-efficacy-of-sodium-glucose-co-transporter-2-inhibitors-versus-dipeptidyl-peptidase-4-inhibitors-in-patients-with-systemic-lupus-erythematosus-and-comorbid-type-2-diabetes-mellitus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-safety-and-efficacy-of-sodium-glucose-co-transporter-2-inhibitors-versus-dipeptidyl-peptidase-4-inhibitors-in-patients-with-systemic-lupus-erythematosus-and-comorbid-type-2-diabetes-mellitus/