Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In Germany, the first etanercept biosimilar was licensed in 2016. In contrast to other European countries there is no uniform recommendation for the prescription of biosimilars. The aim of this study was to compare treatment survival between patients who were switched from the etanercept originator to the etanercept biosimilar SB4 and patients who stayed on the originator treatment.
Methods: We used data of rheumatoid arthritis patients observed in the prospective, longitudinal RABBIT (Rheumatoid Arthritis: Observation of biologic therapy) cohort until November 2018 who were treated with the etanercept originator (oETA) for at least six months. Patients who thereafter were switched to the biosimilar SB4 (bsETA) were matched (1:n) to patients who stayed on the original treatment using prescription time distribution matching [1] to control for survival bias. Matching criteria were sex, time of switch or corresponding duration of originator treatment in non-switchers and age as well as DAS28 at the time of switching or corresponding time point in non-switchers. The retention rates over one year were analyzed using Kaplan-Meier curves.
[1] Zhou Z et al., Am J Epidemiol. 2005.
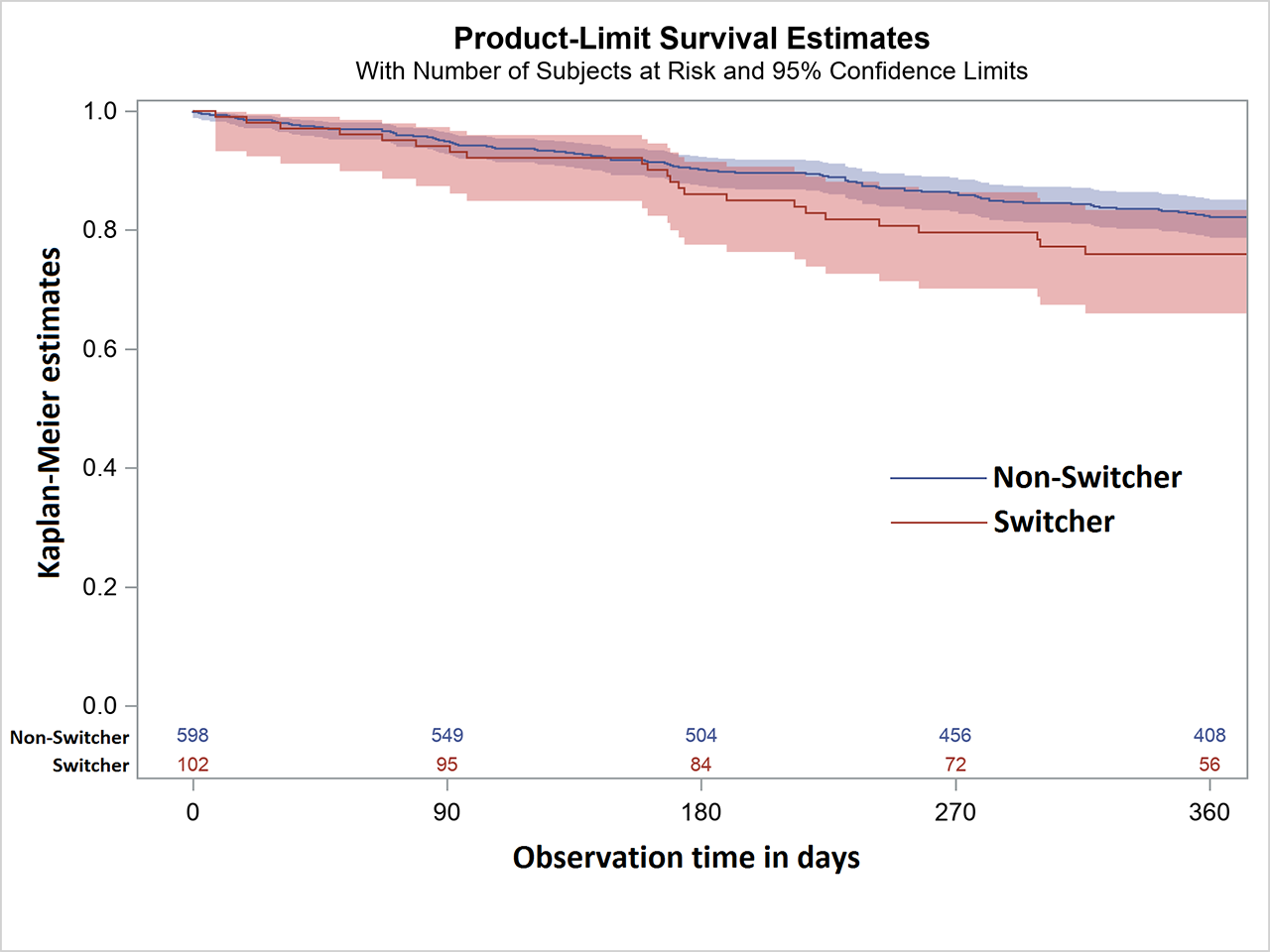
Results: Overall, 1,751 patients fulfilled the inclusion criteria of whom 113 were switched to bsETA. Of these, 102 switchers could be matched to 598 patients who remained on oETA. In both groups, 78% of the patients were female, mean age was about 59 years, DAS28 was 3.2 and physical function as well as numbers of prior biologics were similar. Patients who remained on oETA were more often rheumatoid factor positive (71% vs. 63%), had more erosions (56% vs. 47%) and had more frequently three or more comorbidities (34% vs. 28%) than those who were switched to bsETA. The most common reason for switching was costs (79%). After one year, 23% (n=23) of bsETA patients and 17% (n=99) oETA patients had stopped the respective treatment. The main reason for discontinuation was “adverse events” in bsETA patients (56%, n=13, thereof 2 serious and 1 planned surgery) and “loss of response” in oETA patients (66%, n=65). Kaplan-Meier curves showed similar retention rates over 12 months for bsETA and oETA (figure). Nine bsETA patients were switched back to oETA.
Conclusion: Retention rates of etanercept treated RA patients who were either switched to the biosimilar SB4 or who stayed on the originator are comparable. Only few patients switched back to the originator.
To cite this abstract in AMA style:
Baganz L, Strangfeld A, Herzer P, Krause A, Tony H, Zink A. Comparing Real-world Retention Rates in a Matched Cohort of Rheumatoid Arthritis Patients Who Either Remained on the Etanercept Originator or Switched to a Biosimilar [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparing-real-world-retention-rates-in-a-matched-cohort-of-rheumatoid-arthritis-patients-who-either-remained-on-the-etanercept-originator-or-switched-to-a-biosimilar/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-real-world-retention-rates-in-a-matched-cohort-of-rheumatoid-arthritis-patients-who-either-remained-on-the-etanercept-originator-or-switched-to-a-biosimilar/