Session Information
Date: Tuesday, November 12, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Patient Preferences, Beliefs, & Experiences
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The 2015 American College of Rheumatology (ACR) and 2013 European League Against Rheumatology (EULAR) guidelines for management of rheumatoid arthritis (RA) conditionally recommend consideration of tapering biologic regimen in stable RA patients in remission with due consideration of patients’ values and preferences. Given the paucity of data on perspectives, in this study we sought to explore and compare the patient and providers’ perspectives on long term biologic use and biologic tapering.
Methods: Our study was performed using a survey instrument of adult patients with RA who were being followed at Allegheny Health Network Rheumatology between 01/01/2018 and 12/01/2018. The study included patients who have been on a biologic >6 months and clinically stable per review of their medical record (i.e. had no more than 1 RA flare in last 6 months, no change in DMARD or biologic dose in last 6 months). These patients were contacted to complete a questionnaire via a telephone survey designed to gather qualitative data on their perspectives about long term biologic therapy. A similar questionnaire was administered to their providers. Aggregate responses to each question were analyzed to compare patients and providers’ perspectives, and to identify and predict patients’ likelihood of considering tapering.
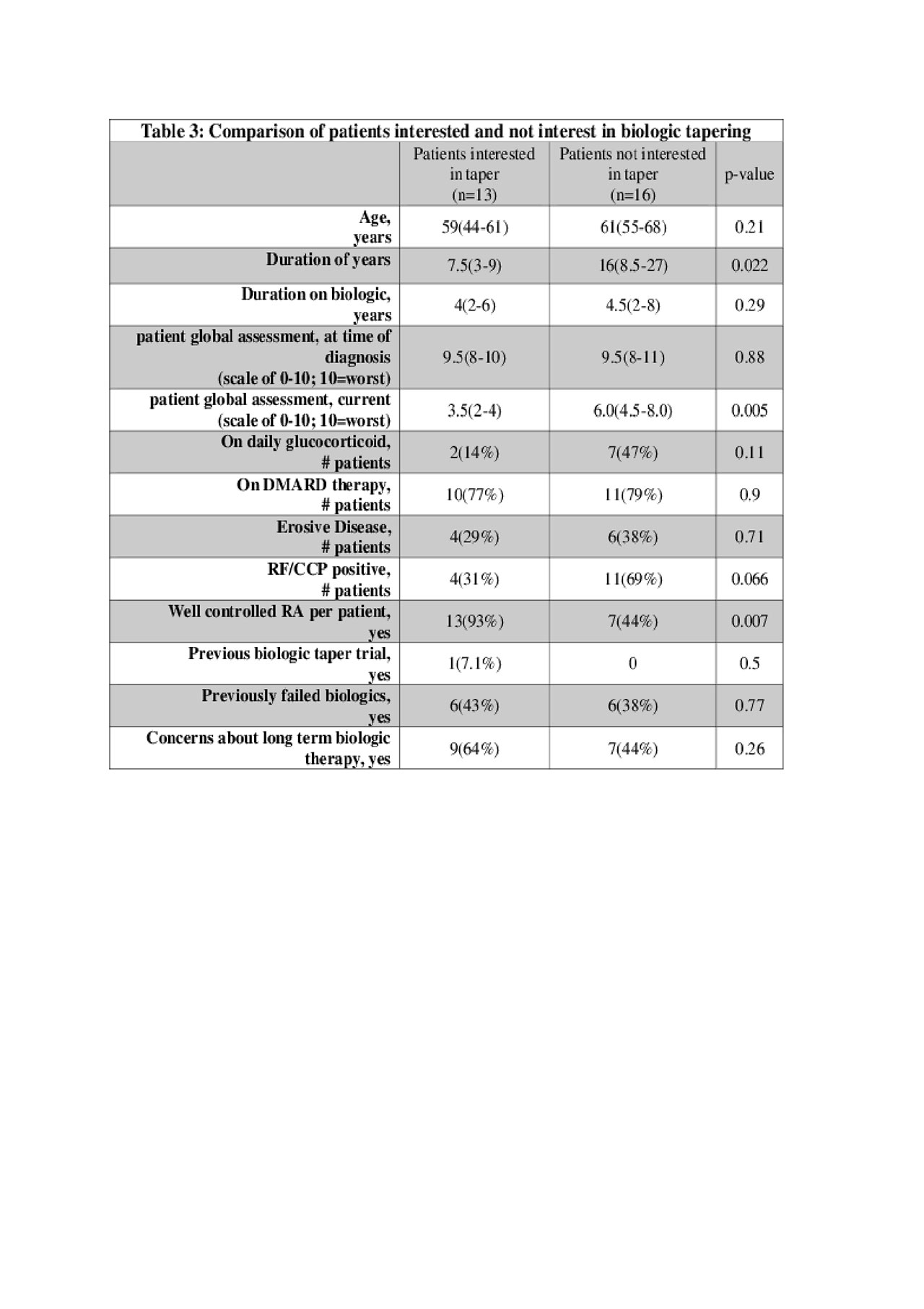
Results: A total of 122 patients met our inclusion criteria. Of these, manual EHR review validated stable RA in 65 patients who were contacted for the telephone survey. 30 patient questionnaires were completed in entirety. Our cohort had the following characteristics: mean age of 55 years, 70% female, 86.7% Caucasian, 53.3% seropositive and 33.3% erosive disease (table 1). 20 of these 30 patients felt their RA was well controlled. 60% of the well controlled patients and 36% of the providers responded that they would consider biologic tapering as an option (table 2). Patients that would consider biologic tapering felt their RA was well controlled with lower patient global assessment scores and had a shorter duration of RA compared to those who did not consider tapering (table 3). The top concern of stable RA patients and their providers about long term biologic therapy was risk of infection and malignancy. Top concern of tapering biologics among both groups was risk of worsening RA (flare, function, pain). Of note, 10 patients (50%) had no concern about being on long term biologic therapy (table 2).
Conclusion: Patients on long term biologic therapy and stable, well-controlled RA were found to be more likely to consider tapering biologic therapy compared to their providers (60% versus 36%) in our survey-based study. Patients and providers shared the same top concerns about continuing or tapering biologic therapy. Patients with well-controlled and a shorter duration of RA were more likely to consider tapering as an option. This information is paramount in designing and implementing our divisional RA Biologic Tapering Initiative (RABTAP), but also to have a more effective and informed shared-decision with patients regarding continuing or tapering long-term biologic therapy in well-controlled RA.
To cite this abstract in AMA style:
Webster P, Wilson N, Payne C, Shields K, Sharma T. Comparing Patient and Provider Perspectives on Long Term Biologic Use and Tapering in Stable, Well-Controlled Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparing-patient-and-provider-perspectives-on-long-term-biologic-use-and-tapering-in-stable-well-controlled-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-patient-and-provider-perspectives-on-long-term-biologic-use-and-tapering-in-stable-well-controlled-rheumatoid-arthritis/