Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
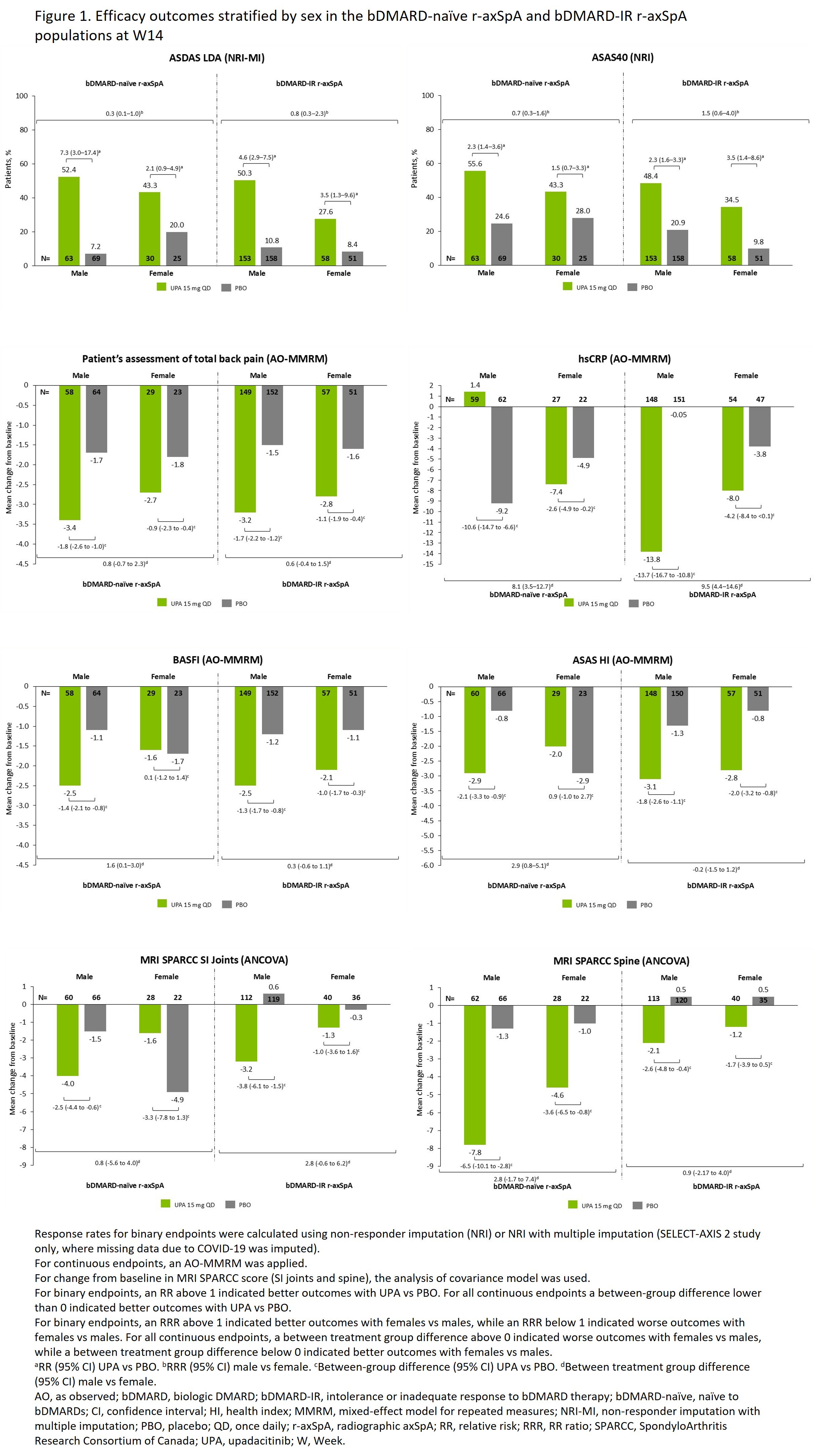
Background/Purpose: Evidence suggests that the treatment effectiveness of TNFis and IL-17is is higher in male (M) vs female (F) patients (pts) with axSpA.1,2 Data comparing the efficacy of JAKis in M and F pts with axSpA are limited. This post hoc analysis of data from 2 phase 3 clinical trials evaluated differences in efficacy between M and F pts with axSpA who were treated with upadacitinib (UPA), an oral JAKi.
Methods: Data were from SELECT-AXIS 1 (NCT03178487; included pts with r-axSpA [historically referred to as AS] naïve to bDMARDs [bDMARD-naïve]) and SELECT-AXIS 2 (NCT04169373; 2 substudies: [1] pts with r-axSpA with an intolerance or inadequate response to bDMARD therapy [bDMARD-IR], [2] pts with nr-axSpA, which included 33% bDMARD-IR pts).3–5 Pts were randomized to UPA 15 mg once daily (UPA15) or placebo (PBO) for 14 (bDMARD-naïve and bDMARD-IR) or 52 (nr-axSpA) weeks (W). In this post hoc analysis, efficacy endpoints were assessed through W14 in all pt populations, and through W52 in the nr-axSpA population. Response rates, stratified by sex (M vs F), were calculated using non-responder imputation (NRI) or NRI with multiple imputation for binary endpoints, and as observed with mixed-effect model for repeated measures for continuous endpoints. Analysis of covariance model was used for mean change from baseline in MRI SPARCC score (SI joints and spine). Efficacy of UPA15 vs PBO was assessed by relative risk (RR) (binary outcomes) or mean between-group differences (continuous outcomes) within each sex (M and F), and the effect of UPA vs PBO in M and F was assessed by the RR ratio (RRR) (binary outcomes) or difference between groups in means (continuous outcomes). Information on the interpretation of RRR and difference between groups in means can be found in the figures.
Results: 920 pts were included, of whom 460 (283/177 M/F) received UPA15 and 460 (290/170 M/F) received PBO. Baseline characteristics were generally similar between sexes, except for greater hsCRP, HLA-B27, and MRI scores in M vs F at baseline. Higher efficacy was seen with UPA vs PBO in both sexes for most endpoints (Figures). At W14, although M generally had numerically better outcomes, results were mostly consistent between M and F, as reflected by RRR or mean difference between differences showing minor differences between both sexes. A trend for higher efficacy outcomes among M vs F pts was most apparent among the bDMARD-naïve r-axSpA population, which had the smallest number of F pts (311/109, M/F). In 3 instances (ASAS40 and ASAS HI in bDMARD-IR r-axSpA, and MRI SPARCC SI joints in bDMARD-naïve r-axSpA), F had numerically better outcomes vs M, based on the RRR or mean difference between differences (Figure 1). In nr-axSpA, there was no strong evidence of differences between M vs F pts at W52 (Figure 2).
Conclusion: UPA generally demonstrated better efficacy vs PBO in both sexes and across all axSpA populations. Efficacy in M vs F pts was generally comparable. References1. Hellamand P, et al. RMD Open. 2023;9:e003325.2. van der Horst-Bruinsma IE, et al. Adv Ther. 2022;39:2806–19. 3. Deodhar A, et al. Lancet. 2022;400:369–79.4. van der Heijde D, et al. Ann Rheum Dis. 2022;81:1515–23.5. van der Heijde D, et al. Lancet. 2019;394:2108–17.
To cite this abstract in AMA style:
Ramiro S, Molto A, Nikiphorou E, Pinheiro M, Urbanik J, Gao T, Chen S, Stigler J, Walsh J, Eder L. Comparing Efficacy of Upadacitinib in Male and Female Patients with axSpA: Results from the SELECT-AXIS 1 and 2 Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparing-efficacy-of-upadacitinib-in-male-and-female-patients-with-axspa-results-from-the-select-axis-1-and-2-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-efficacy-of-upadacitinib-in-male-and-female-patients-with-axspa-results-from-the-select-axis-1-and-2-trials/

.jpg)