Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Guselkumab is an anti-interleukin (IL)-23 monoclonal antibody recently approved for the treatment of psoriatic arthritis (PsA). In two large Phase III trials of patients with PsA (DISCOVER 1&2), guselkumab has shown to be superior versus placebo. In this indication no direct comparison is available between guselkumab and ustekinumab, a monoclonal antibody targeting IL-12 and IL-23. Indirect comparisons based on relative treatment effects versus a common comparator (placebo) only allow for analyses up to week 24 due to cross-over to active arms in available PsA trials. The objective of this study was to indirectly compare joint and skin efficacy of guselkumab versus ustekinumab up to week 52, using pooled patient-level trial data from DISCOVER 1&2 and PSUMMIT 1&2, adjusting for cross-trial population differences.
Methods: Patient level data, including baseline characteristics and outcome data on ACR response and Psoriasis Area Severity Index (PASI) response from the guselkumab arms of DISCOVER 1&2 were pooled with the data from the ustekinumab trials PSUMMIT 1&2. Analyses were performed for bio-naïve and bio-experienced populations separately. Differences in patient characteristics across trial populations were adjusted for using multivariate logistic regression, including gender, age, body mass index, previous TNF use, disease duration, PASI level, number of swollen and tender joints. This method of indirect comparisons allows for analysis of comparative efficacy beyond controlled induction period. Odds ratios resulting from this model were translated into predicted response rates for ustekinumab, assuming same patient population, as enrolled in the guselkumab trial arms.
Results: Majority of baseline characteristics for patients on guselkumab (100mg q8w; 100mg q4w) were comparable to patients on ustekinumab 45/90mg, in both the bio-naïve and bio-experienced group of patients. The probability of reaching a ACR 20 in both the bio-naïve & bio-experienced population was significantly higher for guselkumab vs ustekinumab at weeks 52 for both dosing regimens of guselkumab (bio-naïve ACR 20: q8w OR= 1.88 [1.28;2.76]), q4w (OR= 1.92 [1.29;2.86]; bio experienced ACR20 q8w OR= 2.72[1.17;6.31], q4w OR=4.77 [1.95;11.63]). Similarly, guselkumab was superior to ustekinumab on PASI 90 outcome at week 52 in both bio-naïve & bio-experienced patients with BSA ≥3% at baseline (bio-naïve: q8w OR= 2.59 [1.68;3.99]), q4w OR= 3.19 [2.03;5.00], and bio-experienced q8w OR= 3.96[1.39,11.27], q4w OR=13.10[4.18,41.04]). Figure 1 represents unadjusted pooled DISCOVER 1&2 trial results and estimated proportions of ustekinumab treated patient group achieving ACR 20 in bio-naïve patient group up to week 52 using the method described above.
Conclusion: An adjusted comparison using patient level data from pivotal Phase III studies demonstrates both dosages of guselkumab to be significantly more effective versus ustekinumab in both skin and joint outcomes in both bio-naïve & bio experienced patients up to week 52.
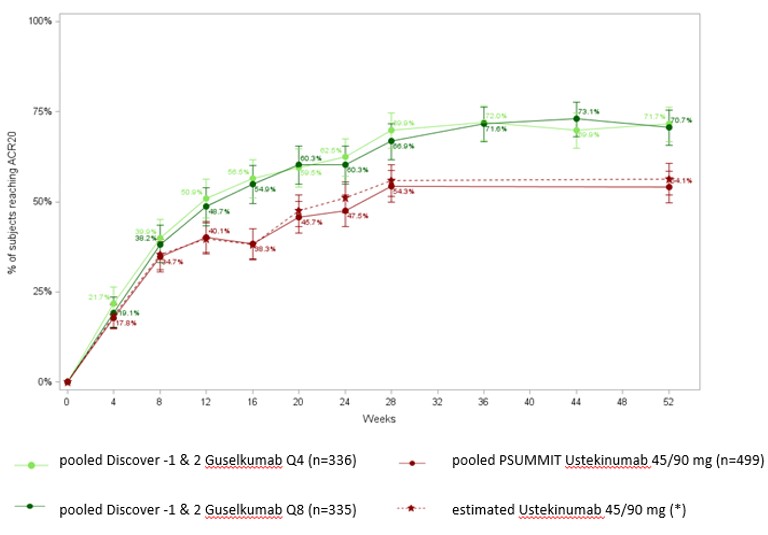 Figure 1. ACR 20 response for guselkumab vs ustekinumab in bio-naïve patients
Figure 1. ACR 20 response for guselkumab vs ustekinumab in bio-naïve patients
To cite this abstract in AMA style:
Diels J, Thilakarathne P, Schubert A, Hassan F, Peterson S, Noël W. Comparing Efficacy of Guselkumab versus Ustekinumab in Patients with Psoriatic Arthritis: An Adjusted Comparison Using Individual Patient Data from DISCOVER 1&2 and PSUMMIT Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/comparing-efficacy-of-guselkumab-versus-ustekinumab-in-patients-with-psoriatic-arthritis-an-adjusted-comparison-using-individual-patient-data-from-discover-12-and-psummit-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-efficacy-of-guselkumab-versus-ustekinumab-in-patients-with-psoriatic-arthritis-an-adjusted-comparison-using-individual-patient-data-from-discover-12-and-psummit-trials/
