Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid Arthritis (RA) is a chronic autoimmune disease affecting synovial joints, often leading to joint destruction if untreated. Disease-modifying antirheumatic drugs (DMARDs), including Janus kinase (JAK) inhibitors like tofacitinib and upadacitinib, are essential for managing RA to achieve remission or low disease activity. However, JAK inhibitors are associated with adverse effects such as venous thromboembolism, malignancy, cerebrovascular and cardiovascular events, infections, and hyperlipidemia. Here is a comparative safety data between tofacitinib and upadacitinib.
Methods: We included RA patients aged ≥18 years, identified by ICD-10 codes, who initiated tofacitinib (n=20,305) or upadacitinib (n=6,281). The index event was the first RA diagnosis with corresponding medication. Propensity score matching (1:1) was performed based on demographics, comorbidities (e.g., diabetes, hyperlipidemia, hypertension), and concurrent medications (e.g., methotrexate, TNF inhibitors). Post-matching, 6,276 patients per cohort were analyzed. Outcomes within five years post-treatment initiation included acute myocardial infarction, cerebral infarction, venous thrombosis/embolism, diverticular disease, pneumonia, herpes zoster, depression, hyperlipidemia, suicidal ideation, lymphoma/leukemia, basal cell carcinoma (BCC), squamous cell carcinoma (SCC), transaminitis, and neutropenia. Patients with prior occurrences were excluded. Risk ratios (RR) with 95% confidence intervals (CI) were calculated.
Results: After matching, each cohort included 6,276 RA patients. Tofacitinib was associated with higher risks of cerebral infarction (RR=1.694, 95% CI 1.29–2.24, P< 0.0001), acute myocardial infarction (RR=2.43, 95% CI 1.858–3.17, P< 0.0001), venous thrombosis/embolism (RR=1.465, 95% CI 1.182–1.818, P=0.0005), diverticular disease (RR=1.373, 95% CI 1.176–1.604, P< 0.0001), pneumonia (RR=1.73, 95% CI 1.472–2.034, P< 0.0001), herpes zoster (RR=1.413, 95% CI 1.157–1.725, P=0.0006), hyperlipidemia (RR=1.24, 95% CI 1.126–1.366, P< 0.0001), and depression (RR=1.453, 95% CI 1.287–1.640, P< 0.0001) compared to upadacitinib. No significant differences were found for suicidal ideation, lymphoid/hematopoietic malignancies, BCC, SCC, transaminitis, or neutropenia as shown in the table below
Conclusion: RA patients treated with tofacitinib have increased risks of cardiovascular events, venous thrombosis/embolism, diverticular disease, pneumonia, herpes zoster, hyperlipidemia, and depression compared to those on upadacitinib. Prospective studies are needed to confirm these findings and investigate underlying mechanisms. Continuous monitoring of patient safety profiles is essential in RA management
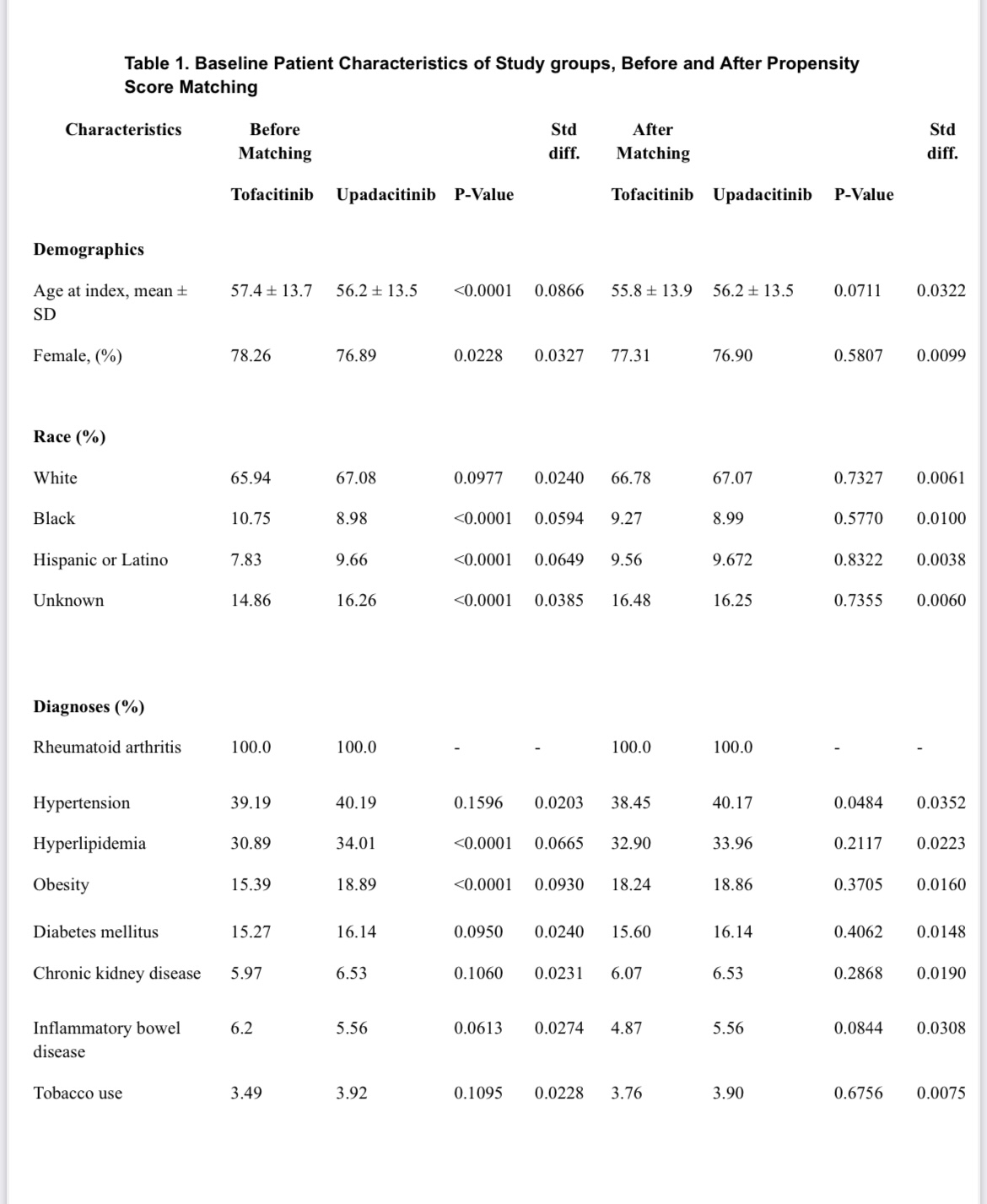 Table 1. Baseline Patient Characteristics of Study groups, Before and After Propensity Score Matching
Table 1. Baseline Patient Characteristics of Study groups, Before and After Propensity Score Matching
.jpg) Table 2. Safety Outcomes in RA Patients: Tofacitinib vs. Upadacitinib.
Table 2. Safety Outcomes in RA Patients: Tofacitinib vs. Upadacitinib.
.jpg) Table 2. Safety Outcomes in RA Patients: Tofacitinib vS. Upadacitinib (continued)
Table 2. Safety Outcomes in RA Patients: Tofacitinib vS. Upadacitinib (continued)
To cite this abstract in AMA style:
Khasho M, Alomari A, Elmusa R, Ali R. Comparative Safety Outcomes of Tofacitinib Versus Upadacitinib in Patients with Rheumatoid Arthritis: A Retrospective Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparative-safety-outcomes-of-tofacitinib-versus-upadacitinib-in-patients-with-rheumatoid-arthritis-a-retrospective-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-safety-outcomes-of-tofacitinib-versus-upadacitinib-in-patients-with-rheumatoid-arthritis-a-retrospective-cohort-study/
