Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: In the ORAL Surveillance trial, cancer risk was higher among patients with rheumatoid arthritis (RA) on tofacitinib, a Janus kinase inhibitor (JAKi), compared to tumor necrosis factor inhibitors (TNFi), in a population enriched for cardiovascular disease risk [1]. However, less is known regarding the comparative safety of non-TNFi biologics relative to TNFi. We assessed the comparative safety of individual non-TNFi and JAKi relative to TNFi for the risk of incident cancer in patients with RA.
Methods: We performed a cohort study using Merative MarketScan databases (2012-2021) of patients with RA identified using ≥1 ICD9/10 codes, age 18-64 years, who initiated treatment with TNFi, non-TNFi (rituximab, abatacept, tocilizumab and sarilumab), or JAKi (tofacitinib, baricitinib) on or after November 2012. Patients with past cancer diagnoses were excluded. We used Cox proportional hazard models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for developing incident cancer (excluding non-melanoma skin cancer) within 2 years of treatment initiation in patients on non-TNFi or JAKi relative to TNFi, adjusting for potential confounders, including demographics, geographic region, year of initiating a biologic, Charlson Comorbidity Index, frailty status measured using claims-based frailty index (2), healthcare utilization within 12 months prior to starting treatment, and proxies for RA severity. Cancer diagnoses were identified using validated administrative algorithms [3]. Patients were allowed to switch from one drug class to the other, allowing a single patient to contribute person-time over different drug classes. Patients were censored if they did fill of a prescription of any of these drugs for >90 days ( >180 days for rituximab), or end of study period (12/31/2021).
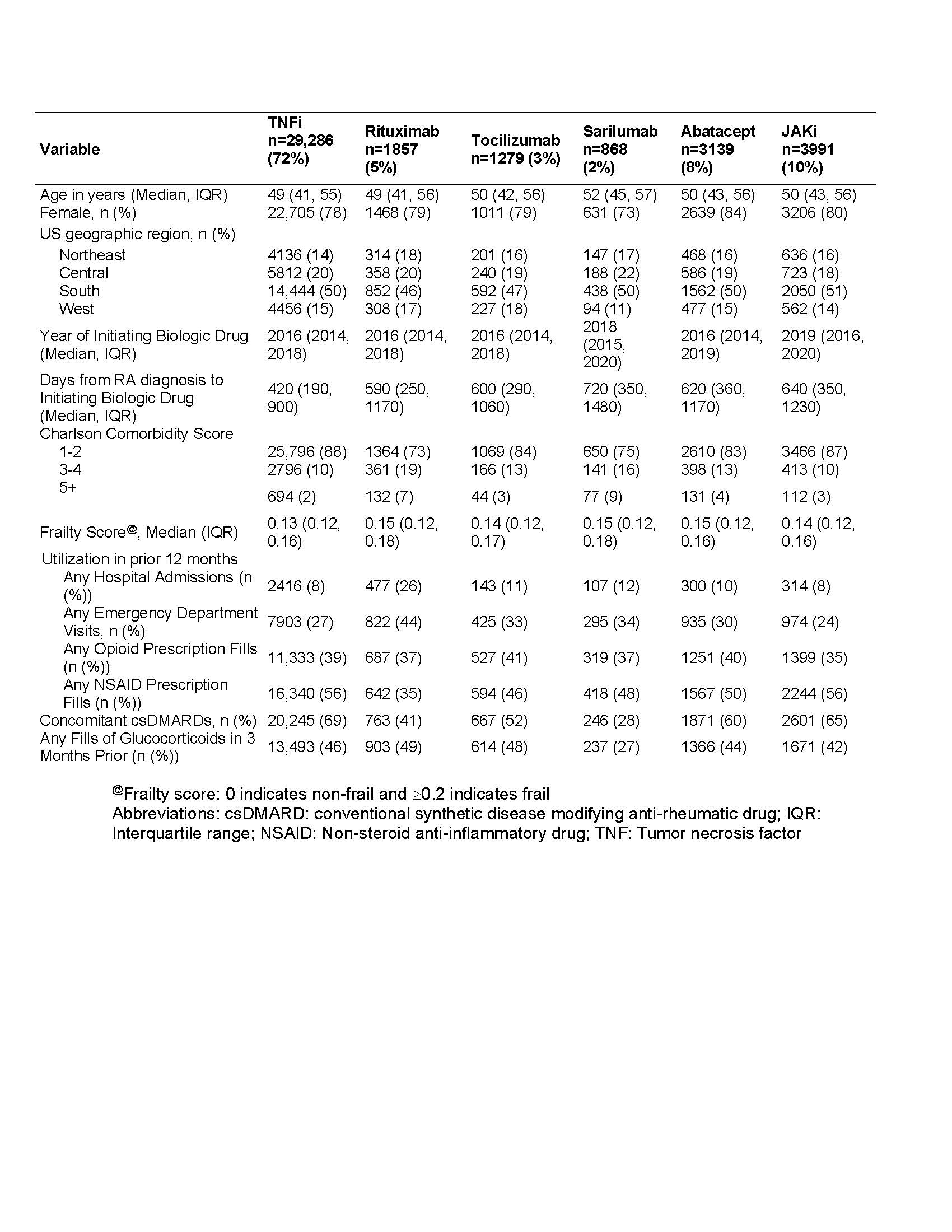
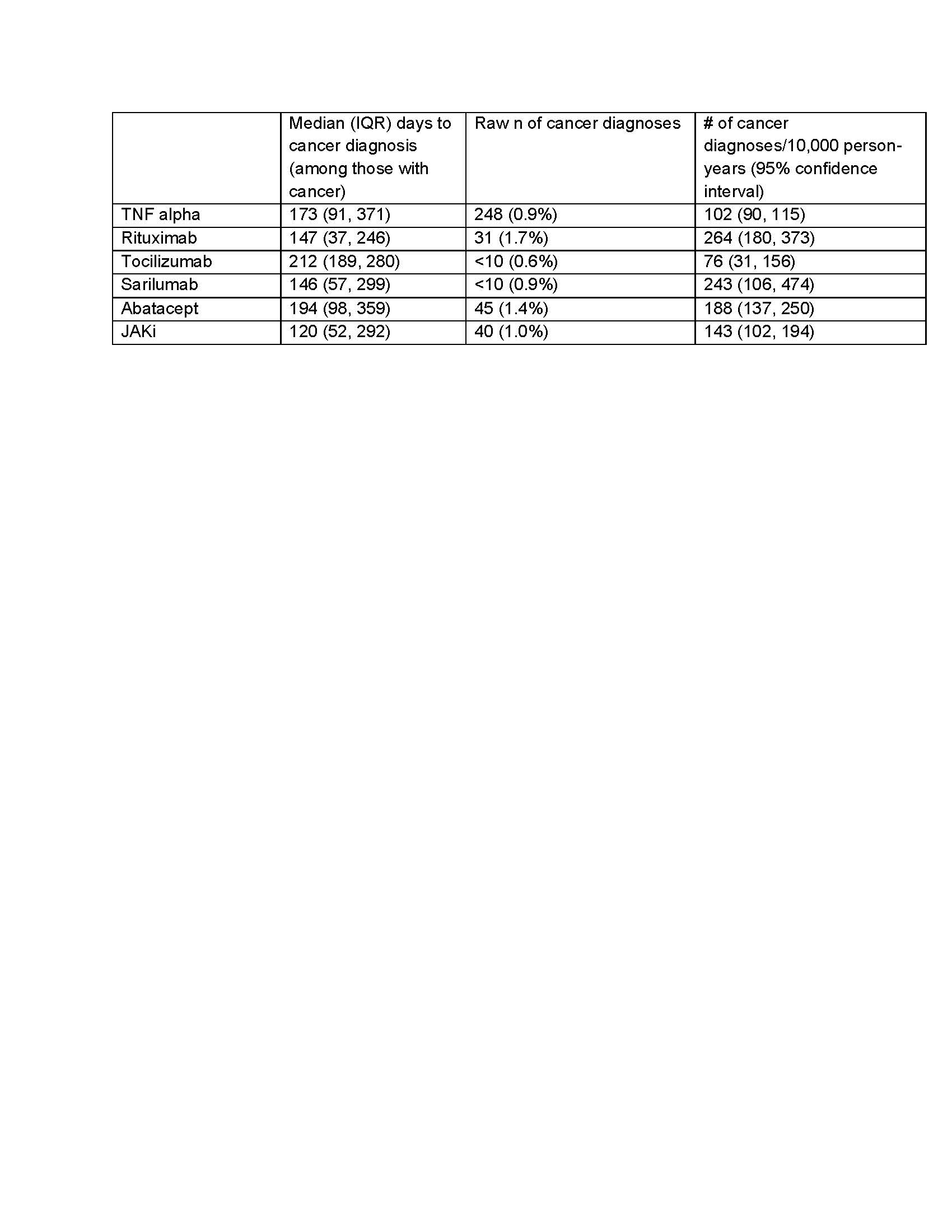
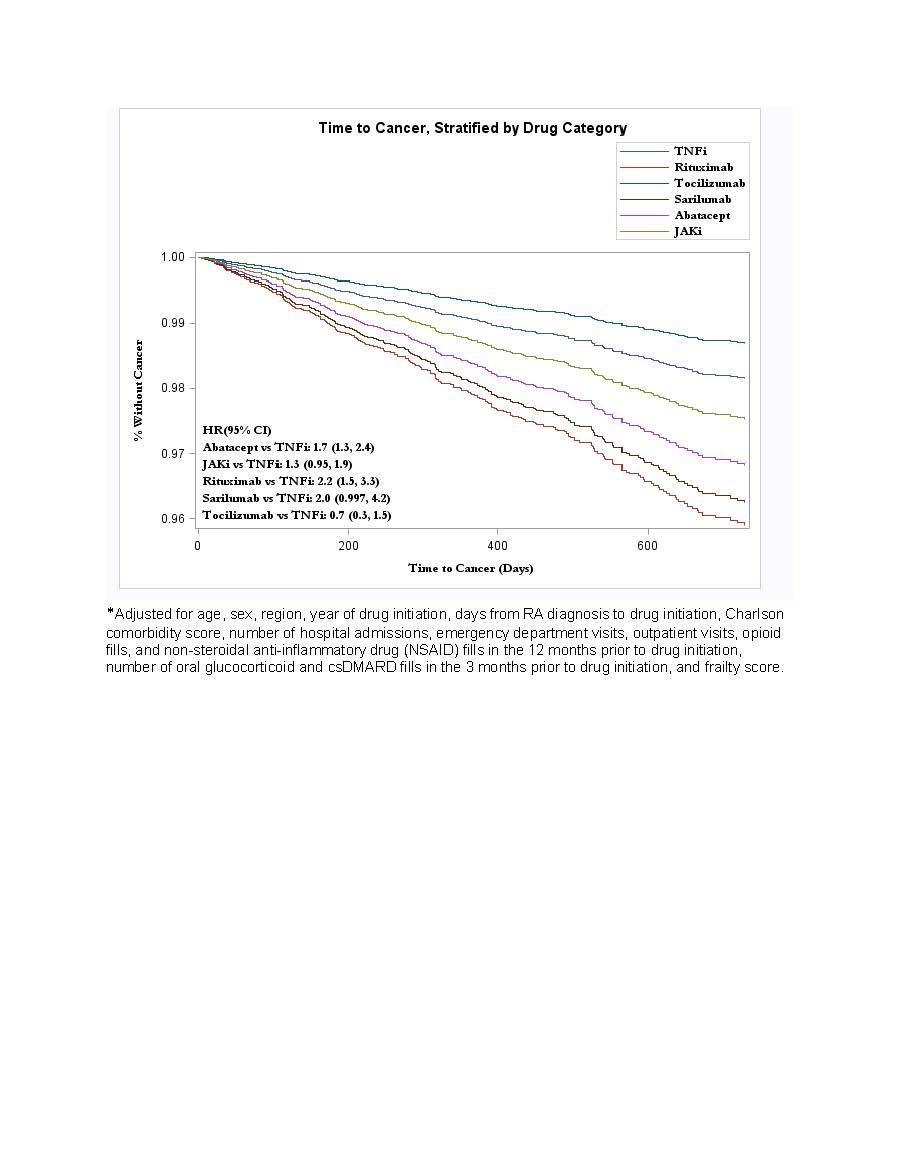
Results: We included 37,026 patients involving 78% female patients with a mean age of 47.6 ± 10.3 years, of whom 72% initiated TNFi, 10% JAKi, 8% abatacept, 5% rituximab, 3% tocilizumab, and 2% sarilumab (Table 1). The mean follow-up time was 360 days for TNFi, 250 days for non-TNFi, and 280 days for JAKi. There were 379 incident cancers observed during follow-up (Table 2). In multivariable models, exposure to rituximab or abatacept had a significantly higher risk of incident cancer (HR 2.2, 95% CI 1.5, 3.3; HR 1.7, 95% CI 1.3-2.4, respectively), compared with exposure to TNFi (Figure 1). While the hazard ratio for incident cancer was higher with exposure to JAKi compared with TNFi, this difference was not statistically significant (HR 1.3; 95% CI 0.9-1.9).
Conclusion: While we observed a lower hazard ratio for incident cancer with exposure to TNFi compared to non-TNFi and possibly JAKi in this generally younger and predominantly female population, potential for residual confounding by indication and the small number of outcomes per drug class limit interpretation of these results. Larger studies with longer follow-up are needed for better comparison of cancer risk between these drug classes.
References:
1. Ytterberg SR, et al: N Engl J Med 2022
2. Kim DH, et al: J Gerontol A Biol Sci Med Sci 2018
3. Setoguchi S, et al: Cancer causes & control : CCC 2007
To cite this abstract in AMA style:
Sendaydiego X, Gold L, liew J, Wysham K, Dubreuil M, Andrews J, Reid P, Liew D, Goulabchand R, Singh A, Hughes G, Pioro M, Sparks J, Jarvik J, Singh S, Singh N. Comparative Safety of Biologic and Targeted Synthetic Disease Modifying Anti-Rheumatic Drugs for Cancer in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparative-safety-of-biologic-and-targeted-synthetic-disease-modifying-anti-rheumatic-drugs-for-cancer-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-safety-of-biologic-and-targeted-synthetic-disease-modifying-anti-rheumatic-drugs-for-cancer-in-rheumatoid-arthritis/