Session Information
Date: Tuesday, November 12, 2019
Title: 5T093: SLE – Clinical V: Emerging Knowledge of Current Treatments (2780–2785)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Human studies examining cardiovascular disease (CVD) risk associated with immunosuppressants (IS) have been limited, but mycophenolate mofetil (MMF) was shown to suppress vascular smooth muscle and endothelial cell proliferation in murine models. In a large SLE Medicaid cohort, we compared CVD event risks among those initiating MMF vs. cyclophosphamide (CYC), used interchangeably for lupus nephritis and severe SLE manifestations, and MMF vs. azathioprine (AZA), used interchangeably longer-term for lupus nephritis or moderately active SLE.
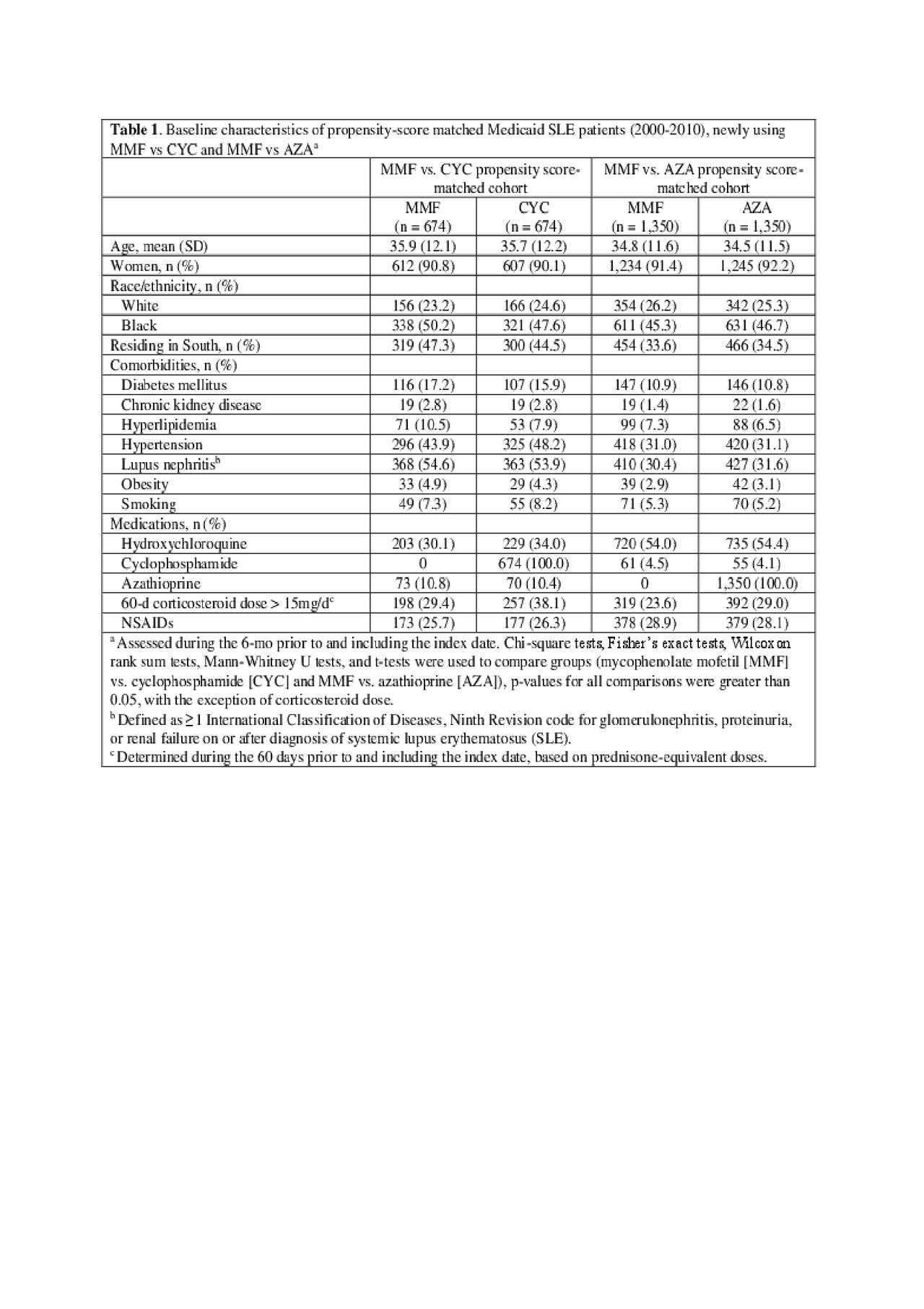
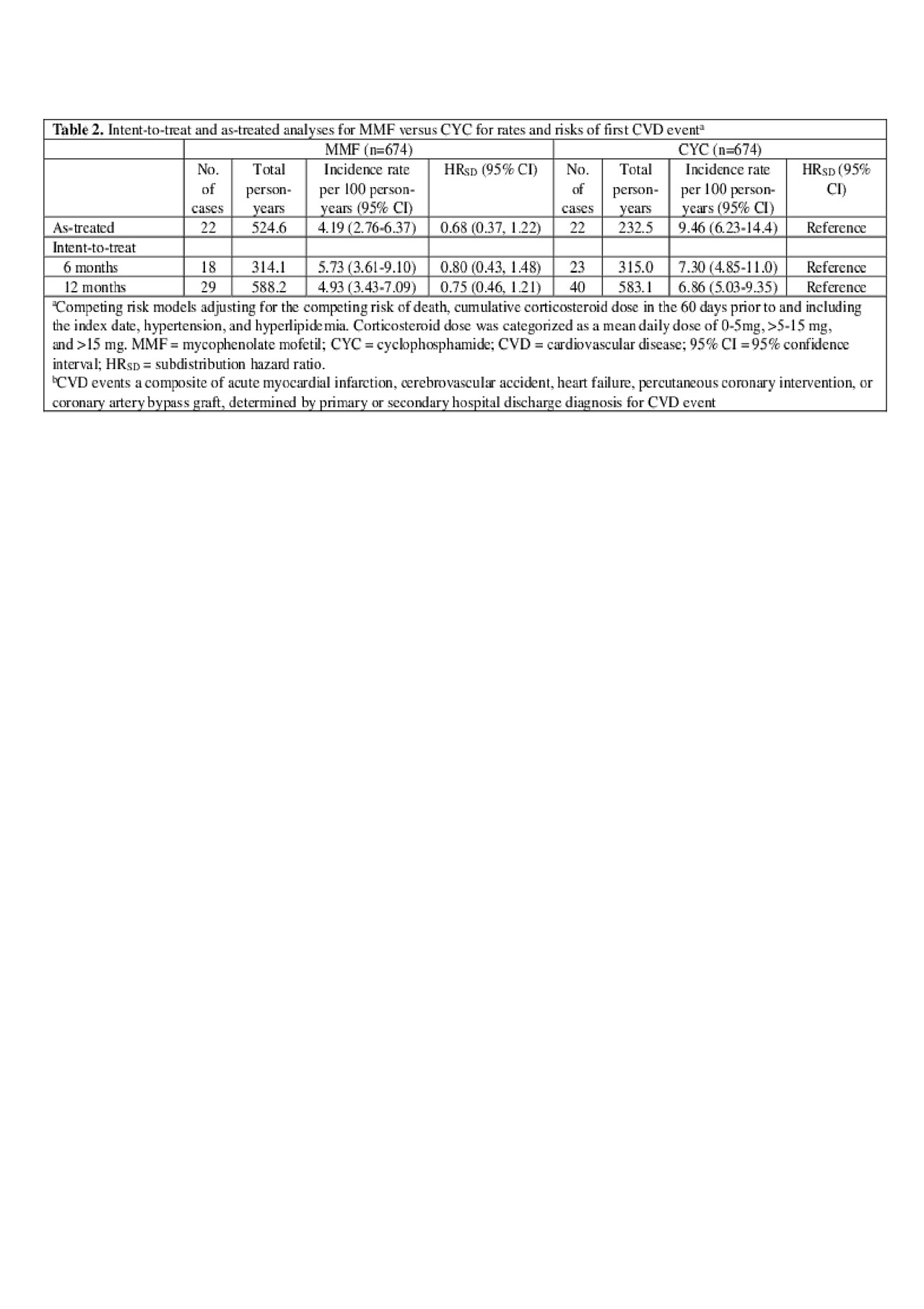
Methods: Within the Medicaid Analytic eXtract (2000-2010; 29 most populated US states), we identified adults ages 18–65 years, with ≥2 International Classification of Diseases, Ninth Revision (ICD-9) SLE codes (710.0) ≥30 days apart. Among them, we identified those filling a new prescription for MMF, AZA, or CYC with continuous Medicaid enrollment ≥6 months prior to first prescription (index date). The CVD event outcome included hospitalized acute myocardial infarction, cerebrovascular accident, heart failure, percutaneous coronary intervention, or coronary artery bypass graft by validated administrative algorithms. Based on sociodemographic, comorbidity, and medication use variables during the baseline (pre-index date), we calculated propensity scores for MMF vs. CYC and MMF vs. AZA. After 1:1 propensity score matching, we estimated incidence rates for first CVD events after drug initiation. Fine-Gray regression models estimated subdistribution hazard ratios (HRSD) for incident CVD events and 95% confidence intervals (95% CIs), accounting for the competing risk of death, additionally adjusting for cumulative corticosteroid dose, hypertension, and hyperlipidemia. Intent-to-treat (ITT) analyses followed subjects from index date for 6 or 12 months, censoring at first CVD event, Medicaid disenrollment or 12/31/2010. As-treated analyses additionally censored at > 60 day gaps in index medication.
Results: We studied 674 propensity-score matched pairs of MMF vs. CYC initiators and 1,350 pairs of MMF vs. AZA initiators. Baseline characteristics were balanced in the matched treatment groups (Table 1). In those with more severe SLE, the point estimate HRSD for first CVD event for MMF vs. CYC (ref.) was < 1, although not statistically significant: HRSD 0.68 (95% CI 0.37, 1.22) in the as-treated analysis, and HRSD 0.75 (95% CI 0.46, 1.21) in the 12-month ITT analysis (Table 2). In those with more moderate SLE, MMF was associated with a reduced risk of CVD vs. AZA (ref). This was non-significant in the as-treated analysis (HRSD: 0.76 [95% CI 0.47, 1.25]), but significant in the 12-month ITT analysis: (HRSD 0.58 [95% CI 0.37, 0.91], Table 3).
Conclusion: In a rigorous head-to-head propensity score matched analysis of SLE Medicaid patients in a large longitudinal study, CVD event risks appeared to be reduced among those taking MMF vs. CYC or AZA, although only significantly lower for MMF vs. AZA in the 12 month ITT analysis. These comparisons adjusted for multiple factors, including corticosteroid use and lupus nephritis. We are obtaining longer Medicaid follow-up data to pursue this interesting finding.
To cite this abstract in AMA style:
Li D, Feldman C, Guan H, Everett B, Kim S, Costenbader K. Comparative Risks of Cardiovascular Disease Among SLE Patients Receiving Immunosuppressive Medications [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparative-risks-of-cardiovascular-disease-among-sle-patients-receiving-immunosuppressive-medications/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-risks-of-cardiovascular-disease-among-sle-patients-receiving-immunosuppressive-medications/